2017年5月,国家局发布了《关于鼓励药品医疗器械创新实施药品医疗器械全生命周期管理的相关政策》征求意见稿(2017年54号文),文中做了以下规定:
开展上市注射剂再评价。按照《药品管理法》相关规定,需要根据注射剂药品科学进步情况,对已批准上市注射剂安全性、有效性和质量可控性开展再评价。生产企业须将批准上市时的研究情况、上市后跟踪研究情况等进行综合分析,开展产品成分、作用机理和临床试验研究,评估其安全性、有效性和质量可控性。力争用5至10年左右时间基本完成已上市注射剂再评价工作。通过再评价的,享受化学仿制药口服固体制剂质量和疗效一致性评价的相关政策。
54 号文中明确要求注射剂要开展一致性评价工作,而且力争10年内完成。但注射剂一致性评价的细则至今尚未给出,注射剂的一致性评价该怎么做?截止2017年5月,已经有多家企业申报了注射剂备案,但注射剂一致性评价工作如何开展任何企业都没底,是BE还是临床?且看 FDA 21CRF.320.22(关于药物体内生物利用度或生物等效性豁免标准), 或许该法案不能告诉我们注射剂一致性评价怎么做,但可以为我们提供一个思路。
既然是注射剂,剂型相同,生物等效性没有实在的意义,如开展临床试验成本又过于高昂,而且临床资源有限,时间花费也很大,可行性不高。对于溶液型注射剂,FDA 在批准ANDA时,只要处方成分和配比一致,可免提交生物等效性证据,这一点也许国家局会采纳。对于药物的安全性方面,口服固体制剂一致性评价的思路或许可以参考,包括杂质谱对比、详细地稳定性、包材相容性研究等。
为了方便大家阅读,我们翻译了21CRF.320.22中关于注射剂、滴眼液、滴耳剂等液体制剂的有关规定,欢迎大家参考。

(a) 任何完整新药申请(NDA)、简化新药申请(ANDA),或补充申请提交之时,如与 320.21(C)一节中的规定有所差异,申请人如想要FDA豁免体内生物利用度评估证据或生物等效性证据,申请人应向FDA提交豁免申请。除本款第(f)项规定外,如药品产品符合本款(b)、(c)、(d)、或(e)项的任何规定,FDA可豁免对生物制剂生物利用度或生物等效性实验数据的要求。
(a) Any person submitting a full or abbreviated new drug application, or a supplemental application proposing any of the changes set forth in 320.21(c), may request FDA to waive the requirement for the submission of evidence measuring the in vivo bioavailability or demonstrating the in vivo bioequivalence of the drug product that is the subject of the application. An applicant shall submit a request for waiver with the application. Except as provided in paragraph (f) of this section, FDA shall waive the requirement for the submission of evidence of in vivo bioavailability or bioequivalence if the drug product meets any of the provisions of paragraphs (b), (c), (d), or (e) of this section.
(b) 对于某些药品,其在体内的生物利用度或生物等效性可能是确定无疑的,FDA应豁免对体内生物利用度和生物等效性数据的要求。如果产品符合下列标准之一,则不必证明该药品在体内生物利用度和生物等效的可能性:
(b) For certain drug products, the in vivo bioavailability or bioequivalence of the drug product may be self-evident. FDA shall waive the requirement for the submission of evidence obtained in vivo measuring the bioavailability or demonstrating the bioequivalence of these drug products. A drug product's in vivo bioavailability or bioequivalence may be considered self-evident based on other data in the application if the product meets one of the following criteria:
(1) 所述药品:
i. 用于肠道外给药的注射溶液剂,或滴眼剂、滴耳剂;
ii. 与已批准的NDA或ANDA具有相同的活性成分和非活性成分,而且各成分的浓度也相同
(1) The drug product:
(i) Is a parenteral solution intended solely for administration by injection, or an ophthalmic or otic solution; and
(ii) Contains the same active and inactive ingredients in the same concentration as a drug product that is the subject of an approved full new drug application or abbreviated new drug application.
(2) 所述药品:
i. 是吸入气体,如吸入治疗或麻醉;
ii. 和已批准的NDA或ANDA具有相同的活性成分、相同的剂型
(2) The drug product:
(i) Is administered by inhalation as a gas, e.g., a medicinal or an inhalation anesthetic; and
(ii) Contains an active ingredient in the same dosage form as a drug product that is the subject of an approved full new drug application or abbreviated new drug application.
(3) 所述药品:
i. 是一种供皮肤使用的溶液、口服溶液、糖浆、酊剂、酏剂,可雾化或喷雾的溶液,滴鼻液,或其他类似的溶液剂型;
ii. 与已批准的NDA或ANDA具有相同的剂型、相同的活性成分浓度,
iii. 不含有非活性成分,或与已批准的NDA、ANDA在处方上存在可能影响药物吸收的变化,或活性部分全身吸收,或显著影响局部作用产品的局部或全身生物利用度,
(i) Is a solution for application to the skin, an oral solution, elixir, syrup, tincture, a solution for aerosolization or nebulization, a nasal solution, or similar other solubilized form; and
(ii) Contains an active drug ingredient in the same concentration and dosage form as a drug product that is the subject of an approved full new drug application or abbreviated new drug application; and
(iii) Contains no inactive ingredient or other change in formulation from the drug product that is the subject of the approved full new drug application or abbreviated new drug application that may significantly affect absorption of the active drug ingredient or active moiety for products that are systemically absorbed, or that may significantly affect systemic or local availability for products intended to act locally.
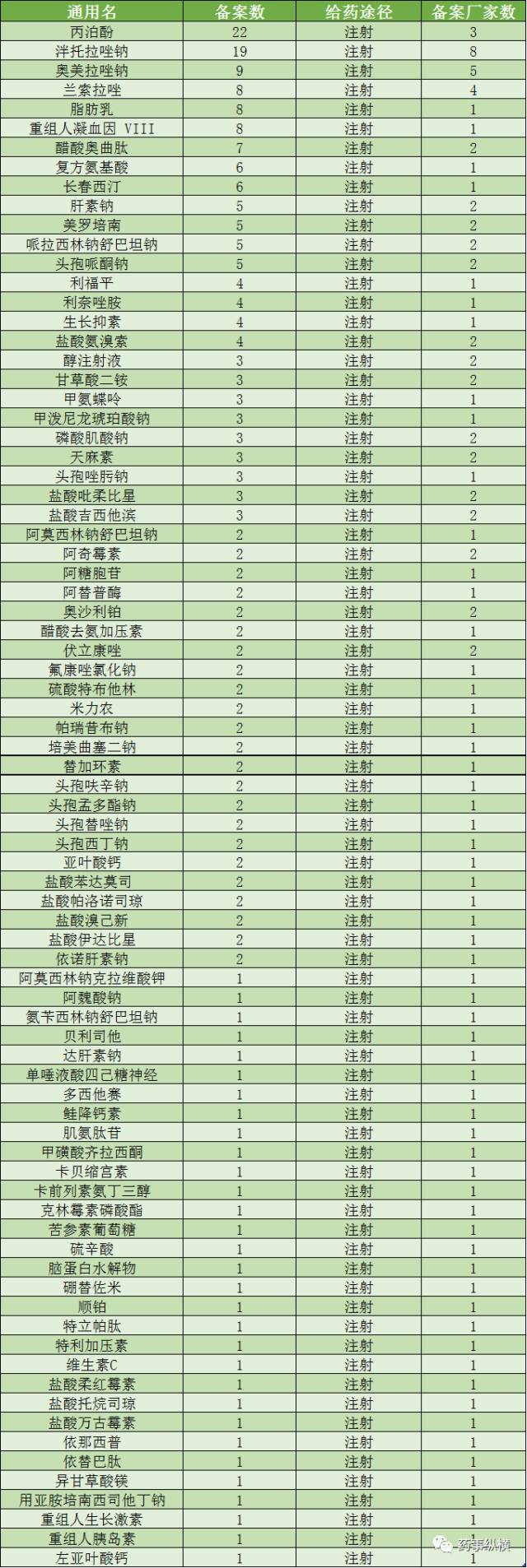
已经提交备案资料的注射剂品种
友情提示:本文仅代表作者本人的意见,只能参考阅读,大家应以国家局规章、指南为准。此外我们不保证我们的翻译完全正确,如有问题请点击原文可查阅21CRF.320.22原文

药事纵横是一个开放,由自愿者组成的团体,现有成员12名,分别为Voyager88,雷诺岛,三分话,Herman,Mzwinsunny,文竹,duke,巉巉之石,ISAL,yhqqqqq,鲁礼炎,占小兵,欢迎有志之士加入我们团队。投稿、合作请加微信442015666。















