
编者按:
上个月底,
加州圣地亚哥抗癌药物开发公司TP therapeutics宣布完成4500万美元C轮融资
,该轮融资由
礼来亚洲风投(Lilly Asia Ventures,LAV)
,OrbiMed Advisors,S. R. One这三家知名风投领投,现有投资人Cormorant Asset Management与SV Tech Ventures也参与了本轮投资。这些资金将用于开发该公司的领先项目TPX-0005,并推进多个临床前项目。TPX-0005会是一款广谱的ALK/ROS1/TRK抑制剂,有效针对多种获得性的突变,从而提供全新的抗癌手段,也是针对肿瘤耐药突变的开发。
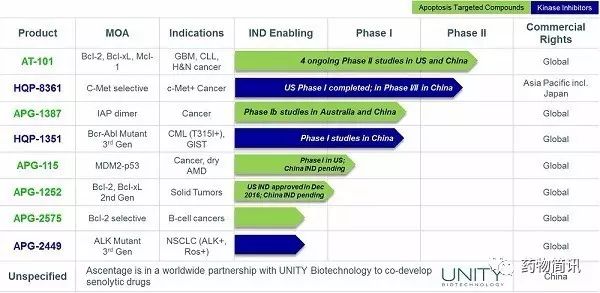
其实,国内针对小分子抑制剂研究的除了去年底完成5亿人民币B轮融资
在这个领域也深耕多年
的亚盛医药。
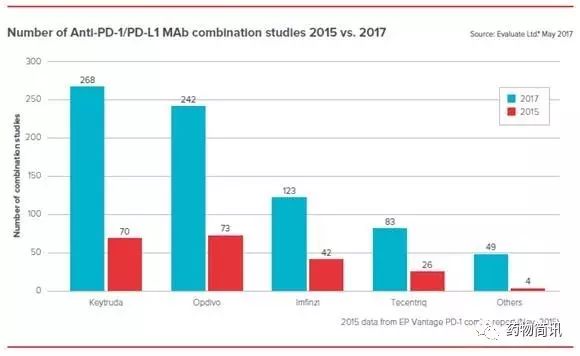
国内也有其他不错的针对肿瘤耐药的项目有待伯乐发掘。在肿瘤免疫疗法火热的时代,许多小分子抑制剂已经琳琅满目,国内很多公司都有,现在如何通过小分子抑制剂与肿瘤免疫抑制剂组合产生协同效应就需要各种运气和临床设计了,尤其是一些肿瘤免疫检查点小分子抑制剂也会是一个很火热的风向标。

Decipheras pharmaceuticals

Decipheras是一家临床阶段生物制药公司主要专注于肿瘤耐药机制研究并且拥有特异性关闭控制平台,今天宣布完成5200万美元C轮融资,该笔融资可以推进公司肿瘤药产品线进入晚期临床开发阶段。融资主要促进
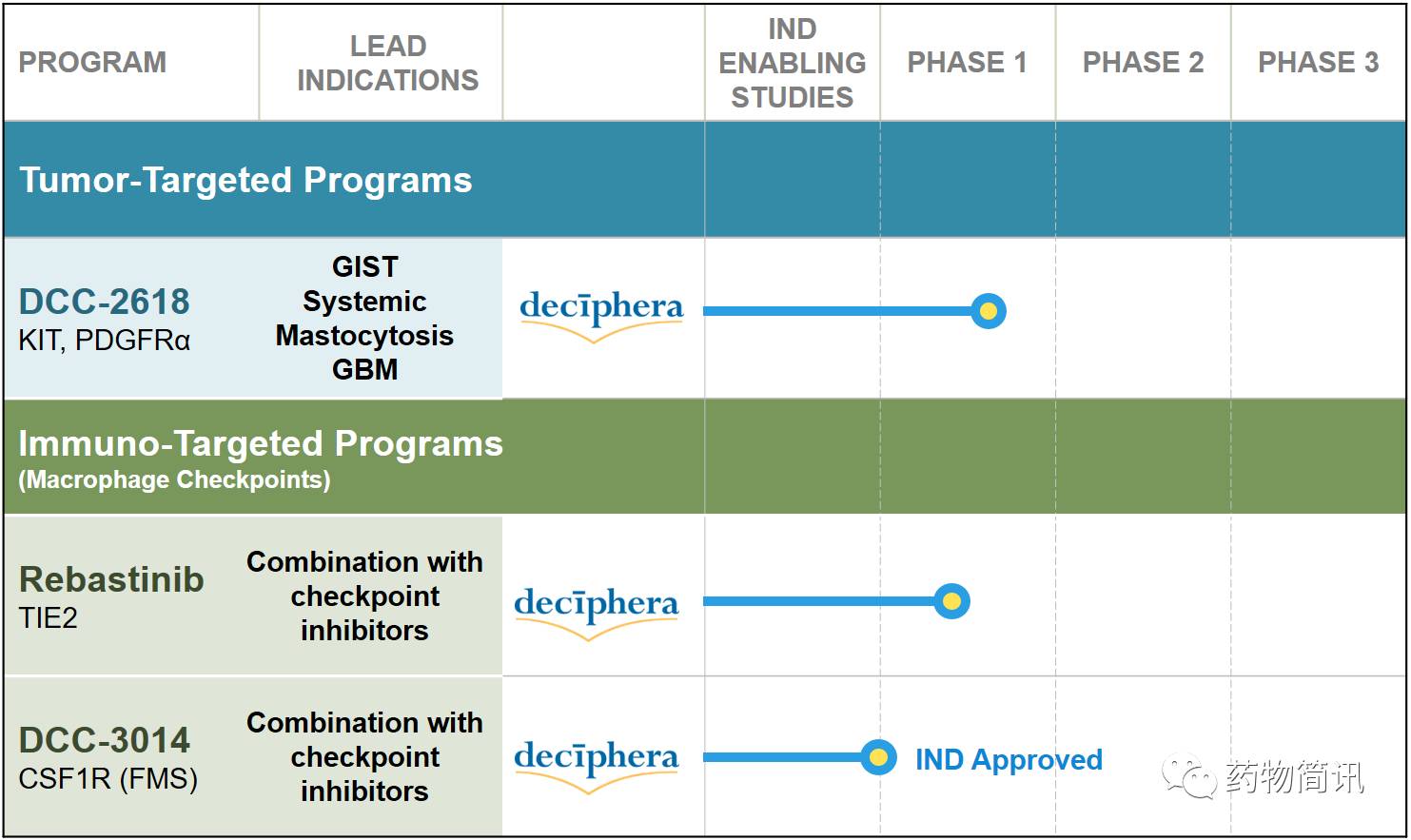
DCC-2618和DCC-3014两个小分子抑制剂进入晚期临床以达到新的疗法克服肿瘤耐药机制改善癌症治疗结果。Deciphera公司的激酶抑制剂可以克服肿瘤耐药从而改善肿瘤响应率和耐受性。这家公司成立于2003年,2015到2016年先后获得了B轮9000万美元融资,之前由礼来和其他投资人投了8500万美元左右,2008年与礼来公司达成4个肿瘤项目合作许可协议。
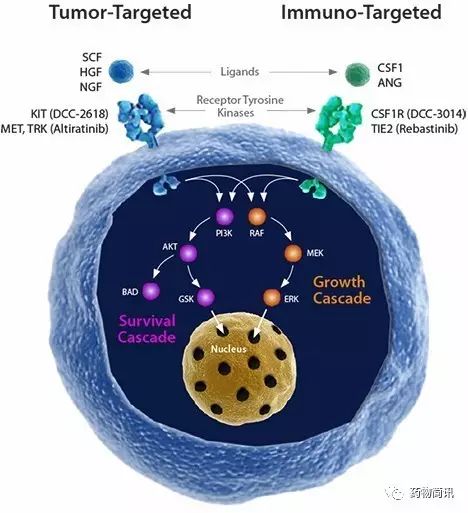
产品线包括肿瘤靶向抑制剂和I/O抑制剂
Deciphera’s
proprietary switch control platform
is uniquely capable of producing advanced kinase inhibitors with distinct profiles and qualities for anti-cancer effects.
WALTHAM, Mass.--(
BUSINESS WIRE
)
--Deciphera Pharmaceuticals, a clinical-stage biopharmaceutical company focused on addressing key mechanisms of tumor drug resistance, today announced that it closed a $52 million Series C financing led by
Viking Global Investors LP, Redmile Group, and Sphera Global Healthcare Fund
and joined by Deciphera’s existing investors including
New Leaf Venture Partners.
The proceeds will enable Deciphera to advance its pipeline of novel oncology products into later-stage clinical development.















