中文导读
世界上许多国家和地区对可饮用水的需求是巨大的。而常规水净化技术,是通过泵使水透过各种昂贵的膜原件(纳滤膜、超滤膜或反渗透膜等),耗费能源,且膜元件损耗较大。有鉴于此,普林斯顿大学的Howard Stone等人发明了一种连续流动的无膜水过滤技术:用CO2净化水,这种方法不仅可以有效去除水中的病毒和细菌,而且环保廉价。
A way to make water potable using carbon dioxide.
THE world’s thirst for clean drinking water is vast and growing. It is also unslaked, particularly in poor countries. The World Health Organisation estimates that more than 660m people rely on what it calls “unimproved” water sources. A quarter of this is untreated surface water. Moreover, even water that has undergone at least some treatment may not be potable. Across the planet, 1.8bn human beings drink water contaminated with faeces. All this polluted water spreads diseases such as cholera, dysentery and typhoid. Every year, more than half a million people die from waterborne diarrhoea alone. As they describe in a paper in Nature Communications, however, Howard Stone of Princeton University and his colleagues have an idea for a new and cheap way to clean water up by mixing it with a substance normally regarded as a pollutant in its own right—carbon dioxide.
There are many existing ways to make water safe to drink, but each has drawbacks. The first step is usually sedimentation: store the stuff in ponds and let as much of the muck as possible drop out under the influence of gravity. But that cannot cleanse water of minuscule, buoyant particles, including many bacteria and viruses, which will not settle. These have to be removed by a second process: filtration.
Filtering water may be done through porous membranes, but that requires pressure, and thus needs costly pumps. Also, the membranes foul quickly, so require frequent replacement. Filtration through beds of sand needs no membranes, but does need chemicals called flocculants to persuade pollutants to coagulate, so that they can be caught by the filter. An alternative, “slow sand” filtration, employs the layers of algae and bacteria that develop on wet sand grains to remove pollutants. It thus requires fewer chemicals. Slow-sand filters must, though, be refurbished regularly. And both sorts of sand filtration miss up to 10% of harmful bacteria.
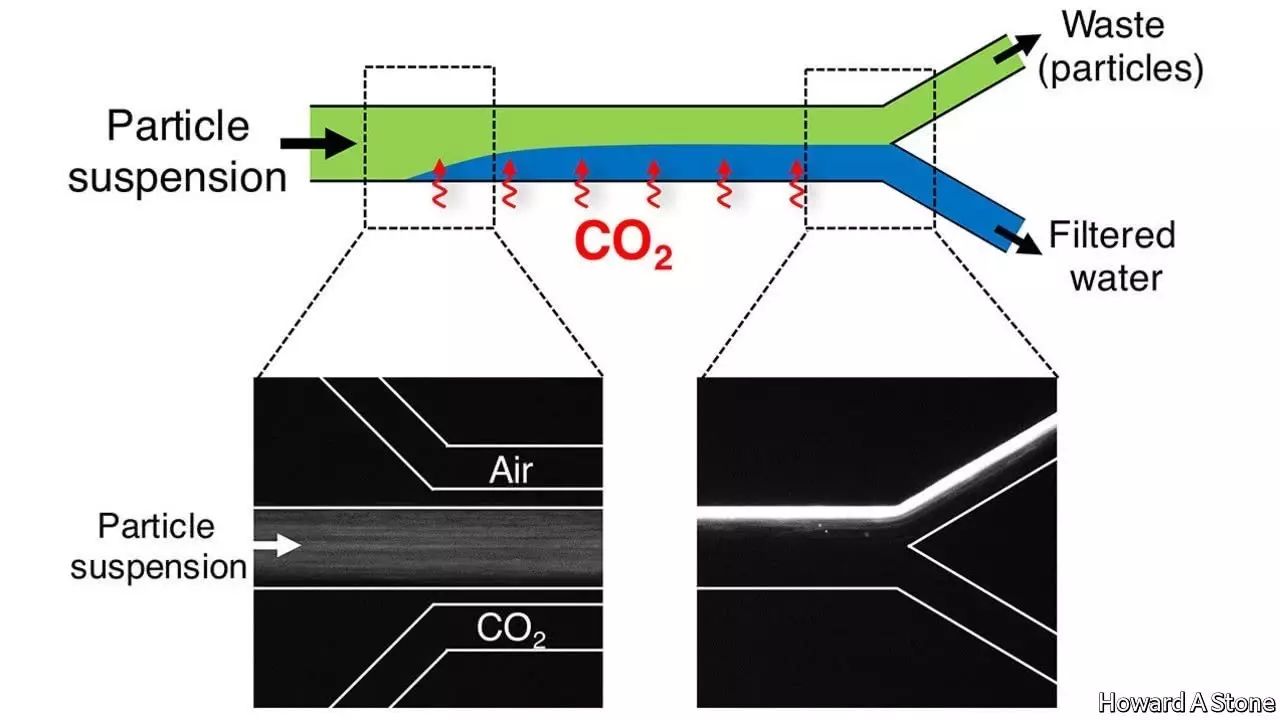
Dr Stone’s alternative is to abandon the idea of filtration altogether. Instead, he plans to apply a phenomenon called diffusiophoresis to the problem. When CO2 and water meet at the liquid’s surface they react to make carbonic acid. This is a solution of hydrogen ions, which are positively charged, and bicarbonate ions, which are negative. The newborn ions then diffuse away from the surface and into the main body of the water. That creates a gradient of ionic concentration perpendicular to the surface. Dr Stone’s insight was that, because the gravity-resistant particles which need to be removed almost always have either positive or negative static-electric charges on their surfaces, their interaction with an ion gradient of this sort, which is itself composed of charged particles, could be used to move them around.
He and his colleagues therefore created an experimental apparatus through which a channel of water flowed in parallel with two channels of gas, one on either side of it, separated from the water channel by gas-permeable membranes. One of the gas channels carried CO2. The other carried air. CO2 thus dissolved into the water on one side of the stream, and out again on the other side, entering the airstream and keeping the gradient constant.
As the team hoped, this arrangement caused suspended particles with positive surface charges to concentrate towards the CO2 side of the water stream, and those with negative surface charges to concentrate towards the air side, leaving the centre of the stream more or less particle-free. In a working system it would simply be a question of splitting the water stream into three as it left the processor, with the two outer branches being recycled and the inner one tapped and piped to consumers.
Dr Stone’s apparatus removed all but 0.0005% of the target particles. And it used less than a thousandth as much energy to do so as membrane filtration would have required. A full-scale version would not need additional chemicals beyond the CO2. And it should, Dr Stone thinks, be easy to maintain.
As to the necessary CO2, he imagines this would come from power stations and other industrial processes, such as cement-making, that produce the gas in large quantities as exhaust. This would restrict diffusiophoretic water plants to industrial cities—but, since such cities are huge sources of demand, that is hardly a problem.
——
May 18th, 2017 | Science and technology | 731 words
















