4月1日,欧盟Eudra GMDP数据库发布了关于中国医药集团中国生物北京生物制品研究所CoV-2灭活疫苗的GMP证书,这是中国历史上首个在欧盟获批使用和GMP认证的疫苗产品:
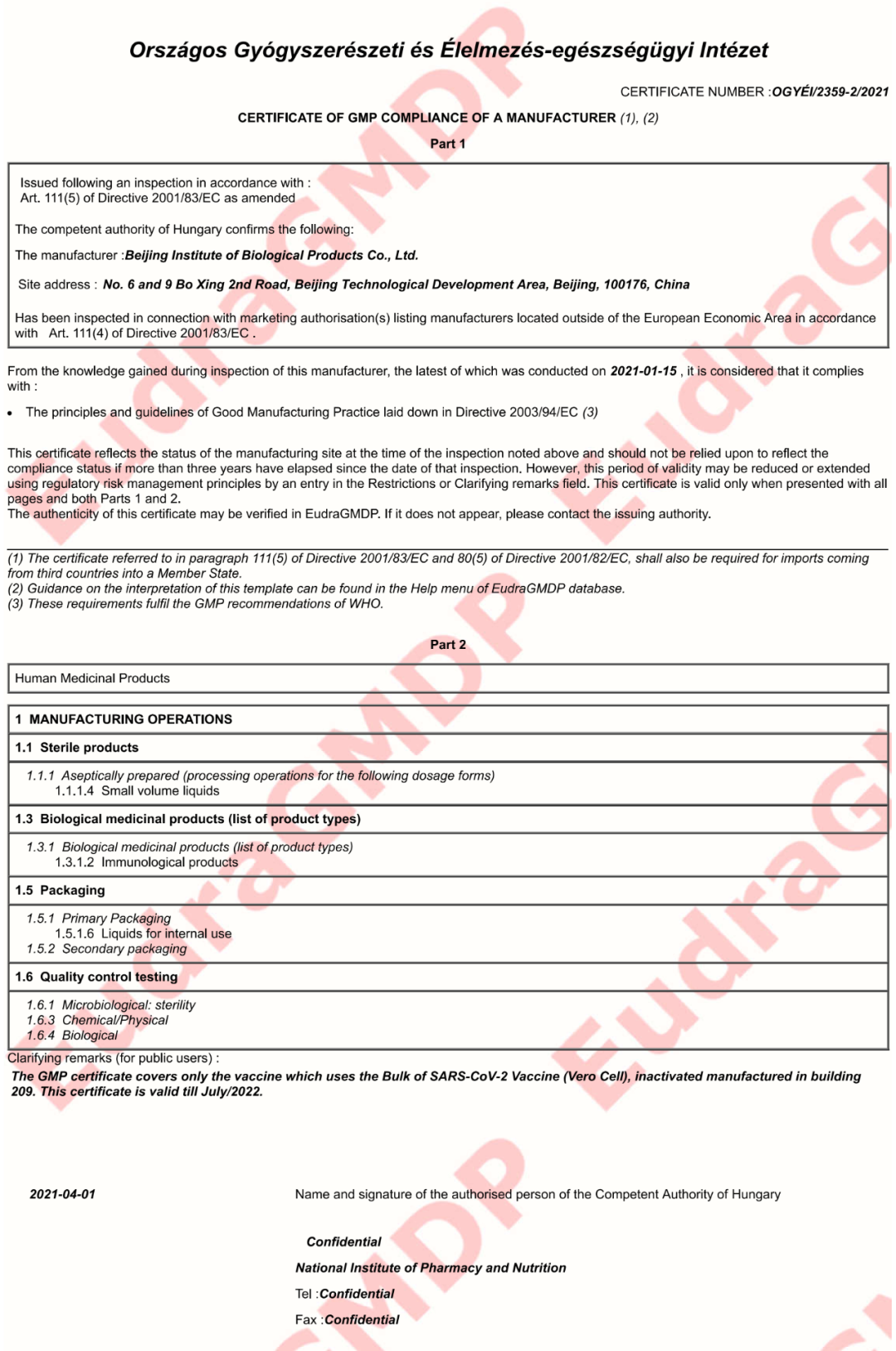
CERTIFICATE NUMBER :OGYÉI/2359-2/2021
证书编号:OGYÉI/2359-2/2021
Issued following an inspection inaccordance with :
根据以下规定进行检查后签发:
Art. 111(5) of Directive 2001/83/EC asamended
指令2001/83/EC第111(5)条修订版
The competent authority of Hungary confirmsthe following:
匈牙利主管当局确认以下事项:
The manufacturer :Beijing Institute ofBiological Products Co., Ltd.
公司名称:北京生物制品研究所有限公司
Site address :No. 6 and 9 Bo Xing 2nd Road, Beijing Technological DevelopmentArea, Beijing, 100176, China
公司地址:北京市技术开发区博兴二路6号、9号,邮编100176
From the knowledge gained during inspection of this manufacturer, thelatest of which was conducted on 2021-01-15 ,it is considered that it complies with :
根据对该制造商的检查(最近一次检查于2021-01-15进行),认为该公司符合:
· The principles and guidelines ofGood Manufacturing Practice laid down in Directive 2003/94/EC (3)
指令2003/94/EC (3)中规定的GMP原则和指南
Clarifying remarks (for public users) :
备注:
The GMP certificate covers only the vaccinewhich uses the Bulk of SARS-CoV-2 Vaccine (Vero Cell), inactivated manufacturedin building 209. This certificate is valid till July/2022.
GMP证书涵盖209车间所生产的散装SARS-CoV-2灭活疫苗(Vero细胞)。本证书有效期至2022年7月。













