Posted on August 13, 2017

Yes, it is time for another medical update!
It is funny… the original intent of this blog (before I published the first post) was for it to be a vehicle to give medical updates to friends and family. Starting with the first post “TAKING THE LATEST TREATMENT PLUNGE” I decided to make the scope of the blog much broader and deeper than that – which I believe happened – to an AMAZING degree!
But in recent months, as my disease has run into more serious twists & turns, the posts have been more & more focused on my own personal medical updates, so in some ways, the blog has transformed partially to its original roots. I have tried hard to include informative explanations as I talk about my medical case however, such that I hope that the blog is still achieving much more than simple medical updates…
Liver Y90-SIRT #2
To begin with – I know many are curious how my Liver Y90-SIRT #2 went a few weeks ago. Y90-SIRT is a fascinating technology that I wrote about in detail here. I mentioned in the post: A CANCER LIFE WEEK: A LITTLE BIT OF THIS, A LITTLE BIT OF THAT) that at the two day mark, the procedure was very well tolerated with basically no side effects.
Right after writing that post, things changed and side effects that some Y90 patients experience hit me… For a couple of weeks, I dealt with a mixture of continuous nausea and continuous fatigue. So dealing with those side effects became a big part of my August – although in “cancer speak” they were “tolerable” (I just got to know my bed and toilet on a stronger relationship level! haha). Thankfully I have been feeling a bit better recently and I think the Y90 side effects are resolving. In terms of treatment, I have not had any since the Y90 procedure on August 2.
Why no treatment?
A mixture of giving my liver/body a break after hitting it with high (localized) radiation with the Y90-SIRT and working out planning for my post-Y90-SIRT treatment! I had mentioned that my next therapy would be an off-label, self-designed, PD(L)-1 combination therapy which both I and my oncologist agreed upon. We finalized plans and the past few weeks we have been working out off-label logistics.
New Treatment Plan Starting on August 22!
On August 22 I’ll get my first infusion of the new treatment plan! I am very excited about it because as I mentioned before, although I certainly wish I didn’t have cancer, it is the greatest research project of my life. As I mentioned, the combination therapy is of my own design (approved by my oncologist) based upon my medical situation and my observations of the CRC experimental therapy landscape over the past few years – which from an empowerment point of view is incredibly cool!
The Treatment Plan
-
Continue FOLFIRI chemo – the purpose here is to continue to control the aggressive growing tumors in my liver. In general they appear to be sensitive to FOLFIRI/Erbitux and I am placing the bet that FOLFIRI is doing the heavy lifting in that combination. The Y90-SIRT appears to have taken care of the biggest, most dangerous and a large proportion of my liver tumors – but not all – so the liver still needs aggressive containment with systemic therapies. We bought some significant breathing room with the Y90-SIRT’s but not 100% containment.
-
-
Add in the PDL1 inhibitor “atezolizumab” (Tecentriq) – this is to attempt to bring my immune system into play and attempt to control some slow growing tumors in other parts of my body which are FOLFIRI-resistant. I have met a number of patients where chemo + PD(L)1 inhibition was quite active. Although anecdotal, it makes this combination have some successful clinical precedent. There are numerous clinical trials on-going which combine chemo with PD(L)1 inhibitors based upon pre-clinical data which shows synergy – e.g. perhaps via neoantigen release upon cytotoxic killing of cancer cells.
-
-
Swap in Bevacizumab (Avastin) for current Cetuximab (Erbitux) – I have been on Erbitux since March. Previous to that I was on Avastin. I swapped out Avastin (which appeared to be working) primarily to facilitate the various surgeries and procedures I did in the spring/summer. Those are now done and in preclinical models Avastin is more synergistic with PD(L)1 inhibitors than Erbitux – so it is time to switch back!
-
-
Continue Probiotics – In preclinical models they are synergistic with PD(L1) inhibition and in an ever-growing pile of clinical data, the importance of the gut microbiome on the systemic immune system is being observed!
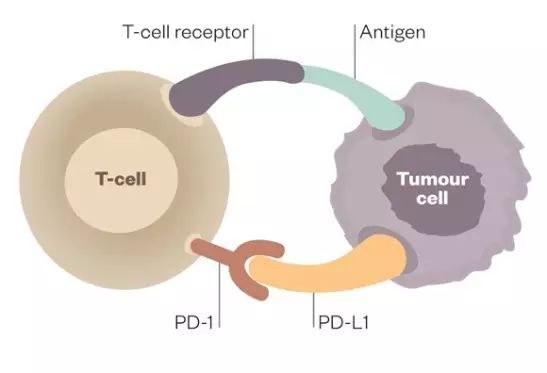
As I said, the first dosing of this triplet therapy is on August 22nd! Wish me luck as I leave the exit ramp of FDA-approved therapies and enter the off-roading world of experimental treatment strategies as my primary treatments! A reminder: based upon the aggressiveness of my disease, my inability to fly to a different trial site, my melanoma etc – for medical & logistical reasons (and there being a reasonable off-label strategy to try): an off-label strategy made more sense for me right now than a clinical trial…
Speaking of Clinical Trials…
Although I am hopeful and optimistic that the above described PD(L)1 combination strategy will be efficacious – I also have to hope for the best and plan for the worst. I have often used the analogy of having multiple treatment plan planes lined up on my taxiway, with their positions being swapped as medical and logistical situations change.
The next plane on my taxiway (which if the current planned treatment doesn’t work, I’ll need to activate in the fall) is a clinical trial currently being set up at UCSD. It uses a high “pulse dosed” EGFR inhibitor “erlotinib” (Tarceva). This means that a very high dose of erlotinib is given – but only for a few days at a time. Based upon data I have seen, this looks like a very promising treatment strategy to me and I am excited to participate in the trial!

















