
欢迎点击「PaperRss」↑关注我们!
美国美国食品和药物管理局最近给第一个基因疗法的批准铺平了道路,(
Therapy that targets disease-causing mutations could become the first of its kind approved for use in the United States.
)
2017
年
10
月
12
日,一个外部专家小组投票一致认为,该基因疗法对一种遗传性失明治疗的价值超过风险,最终决定
voretigene neparvovec(Luxturna)
预计将在
2018
年
1
月
12
日。
此前
voretigene neparvovec
已收到
FDA
突破性疗法和孤儿药资格认证,正在被开发作为由
RPE65
基因突变引起的
遗传
性视网膜疾病(
IRD
)的潜在治疗方案。
遗传
性视网膜疾病是一种致盲性罕见遗传病,由基因
RPE65
功能丧失引起。
RPE65
基因突变患者视网膜感光细胞(色素细胞)会逐渐失去功能并坏死,最终导致视觉功能的完全丧失。
Luxturna
是宾夕法尼亚州
Spark Therapeutics
公司设计并用来治疗那些有两个称作
RPE65
基因缺陷的患者。
Luxturna
是应用腺相关病毒或非致病感冒病毒为载体,将
RPE65
序列编码入病毒载体,再注射到患者视网膜内得以蛋白表达,这样便有望促进视网膜感光细胞的存活和功能,有效将光信号转换成电信号,最终患者的眼睛可以恢复视觉感光。
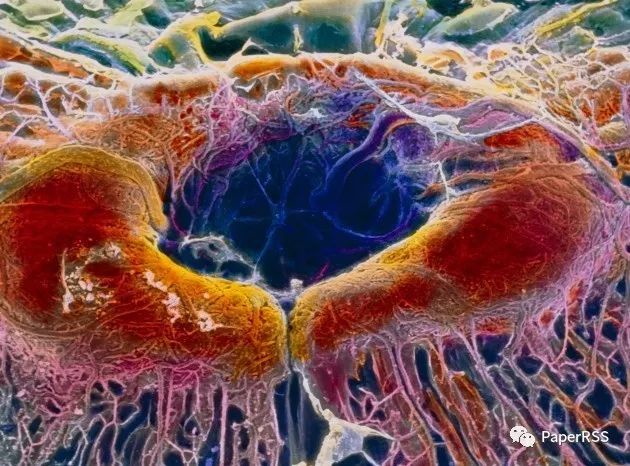
P. Motta/Dept. of Anatomy/University “La Sapienza”, Rome/SPL
The US government is considering whether to approve a gene therapy to prevent the degradation of cells in the retina (shown here in an image from a scanning electron microscope).
这一疗法如果获得批准,将给美国药品市场带来丰厚的利润,这也是基因治疗研究人员等待了几十年的回报。加州斯坦福大学遗传学家马克·凯说,这将是第一次批准基因疗法,会开启基因治疗新时代。
31
人随机对照试验结果发现,患者的视力障碍得当明显改善。这个疗效长达两年之久。对照组显示没有任何改善。这足以说服
FDA
顾问委员会疗法的好处大于风险。这是一个经历了
20
年漫长等待的一个结果。
波士顿儿童医院首席科学官
David Williams
说,在
1990
年代早期,基因治疗技术红地发紫,当时每个年轻学者都希望从事这方面的研究。然后是一个参与基因治疗临床试验年轻病人死亡,让人们意识到基因疗法可以导致免疫失调患者变成白血病。投资者放弃了基因治疗,一些学者也远离这一领域。尽管
2012
年欧洲监管机构批准了一个基因疗法,治疗一种可导致严重胰腺炎基因缺陷疾病,但是许多人怀疑这种治疗的效果。市场药品许可证将于
10
月
25
日到期,公司已经宣布不会更新。
有人甚至认为基因治疗属于伪科学。但一些研究人员坚持在这个领域,努力优化技术提高细胞导入外来基因的效率。临床试验开始显示基因治疗的临床效果,制药公司也逐渐对开发罕见遗传疾病治疗感兴趣,投资者又渐渐地回来了。现在对基因治疗载体等的需求非常高,研究人员为了获得特定试剂甚至需要等待
1
年半到
2
年时间。
估计在过去几年中,基因治疗临床试验的疾病类型已经有很多,如血友病、镰状细胞病和
Wiskott-Aldrich
综合症等。
10
月
4
日,
Williams
和他的同事发表肾上腺脑白质营养不良的基因治疗试验结果。
17
名受试男孩患者在治疗
2
年期间有
15
名病情没有继续恶化。
8
月
30
日,
FDA
批准了第一个免疫细胞工程对抗癌症的基因疗法,但是癌症治疗和这种基因疗法完全不同,癌症治疗并不是针对特定的致病基因突变。而是从患者身体内获得免疫细胞进行基因工程改造。这就是为什么称
FDA
批准
voretigene neparvovec
将是一个里程碑。因为这才是真正意义的基因治疗。
斯坦福大学儿科血液学学者
MatthewPorteus
说,人们是如此兴奋。但
Spark Therapeutics
公司也强调,这一代基因疗法仍然存在不足。虽然治疗似乎改善视力,现在仍不清楚病毒表达正常
RPE65
基因会继续多久。马克·凯说,这不是治愈的概念,同样大脑退化疗法似乎减缓疾病对大脑的影响。但预计不会治疗其他部位的症状。在基因治疗技术道路上,仍然有许多困难等待者科学家去克服。
Nature报道原文:
Advisers to the
US Food and Drug Administration (FDA) have paved the way for the agency’s first approval of a gene therapy to treat a disease caused by a genetic mutation.
On 12 October, a panel of external experts unanimously voted that the benefits of the therapy, which treats a form of hereditary blindness, outweigh its risks. The FDA is not required to follow the guidance of its advisers, but it often does.
A final decision on the treatment, called voretigene neparvovec (Luxturna), is expected by 12 January.
An approval in the lucrative US drug market would be a validation that gene-therapy researchers have awaited for decades
. "It’s the first of its kind," says geneticist Mark Kay of Stanford University in California, of the treatment. "Things are beginning to look more promising for gene therapy."Gene replacement
Engineered cell therapy for cancer gets thumbs up from FDA advisersPromising gene therapies pose million-dollar conundrumLeukaemia success heralds wave of gene-editing therapiesMore related stories
Luxturna is made by Spark Therapeutics of Philadelphia, Pennsylvania, and is designed to treat individuals who have two mutated copies of a gene called RPE65. The mutations impair the eye’s ability to respond to light, and ultimately lead to the destruction of photoreceptors in the retina.
The treatment consists of a virus loaded with a normal copy of the RPE65 gene. The virus is injected into the eye, where the gene is expressed and supplies a normal copy of the RPE65 protein.
In a randomized controlled trial that enrolled 31 people, Spark showed that, on average, patients who received the treatment improved their ability to navigate a special obstacle course1. This improvement was sustained for the full year during which the company gathered data. The control group, however, showed no improvement overall. This was enough to convince the FDA advisory committee that the benefits of the therapy outweigh the risks.
Long road
That endorsement is an important vote of confidence for a field that has struggled over the past 20 years. In the early 1990s, gene therapy was red hot, says David Williams, chief scientific officer at Boston Children’s Hospital in Massachusetts. "You couldn’t keep young people out of the field," he says. "Everyone wanted in." Then came the death of a young patient enrolled in a gene-therapy clinical trial, and the realization that a gene therapy used to treat children with an immune disorder could cause leukaemia.
Investors backed away from gene therapy, and some academics grew scornful of it. Although European regulators approved one such therapy in 2012, for a condition that causes severe pancreatitis, many doubted that it worked. (The company that makes it has announced that it will not renew its licence to market the drug when it expires on 25 October.) "You’re too smart to work in this field," a colleague told Kay. "It’s a pseudoscience."But some researchers kept plugging away at the problem, improving the vectors that shuttle genes into human cells. Over time, new clinical trials began to show promise, and pharmaceutical companies became more interested in developing treatments for rare genetic diseases. Gradually, investors returned.
Now, demand for gene-therapy vectors is so high that suppliers are oversubscribed, and researchers have to wait between 18 months and 2 years to get some of the reagents that they need for clinical studies, says Williams.
Measured expectations
In the past few years, gene therapies have shown promise in clinical trials for a range of diseases — including haemophilia, sickle cell disease and an immune disorder called Wiskott–Aldrich syndrome. On 4 October, Williams and his colleagues published results of a gene-therapy trial to treat cerebral adrenoleukodystrophy (ALD), a devastating and sometimes fatal disorder that affects the nervous system and adrenal glands2. Disease progression was halted for the roughly 2-year duration of the study in 15 of 17 boys who were treated.
The FDA approved its first gene therapy, a treatment in which immune cells are engineered to combat cancer, on 30 August. Unlike Spark’s therapy, the cancer treatment does not target a specific disease-causing mutation, and is administered to immune cells that are removed from the body, engineered and then reinfused.
That is why researchers say that an FDA approval for voretigene neparvovec would be a landmark. "The general concept of gene therapy is replacing or compensating for a missing gene, and that’s what this does," says Matthew Porteus, a paediatric haematologist also at Stanford. "People are so excited."But Spark’s treatment also highlights the limitations of this generation of gene therapies. Although the treatment seems to improve vision, it is still unclear how long the virus will continue to express the normal RPE65 gene — and thus how long its effects will last. "It isn’t a cure," says Kay.
Similarly, the cerebral ALD therapy seemed to slow the effects of the disease in the brain, but is not expected to treat symptoms in other parts of the body, which can emerge later in life.
"I think we still need to have major improvements in the technology before we’re going to be able to cure these diseases," says Kay. "But along the way there may be treatments that help make improvements."
Nature doi:10.1038/nature.2017.22819


如果您觉得这篇文章不错,请转发到您的朋友圈吧!
( 免责声明: 本文中的部分信息援引自网络。本公众号发布的图文一切仅为分享交流,并不代表本公众号的观点。所有援引自网络的部分,其版权归原作者、原公号或原网站所有,如有涉及版权敬请及时告诉我们,定将及时删除或妥善处理。)

长按下面二维码,关注我们!
觉得不错点个赞吧!





