近几天来,大家都在疯传ICMJE的新规则:Data Sharing Statements for Clinical Trials — A Requirement of the International Committee of Medical Journal Editors。
在新规则下,医生还能不能好好发SCI论文了?
“
目前国内医学SCI投稿最大的障碍与ICMJE的新规则:动物伦理、临床试验伦理及临床试验注册”
。我们在规划SCI前,一定要将非学术的问题解决好!
我们主要从三个方面来讨论。最后讨论ICMJE的最新规则。
1、动物伦理和临床试验的伦理
:课题设计完成,在开始实验/试验之前,一定要拿到
动物伦理和临床试验的伦理
。伦理问题,尤其是临床试验的伦理,是比较重要的,一定要有。当然,目前关于动物伦理,也已有SCI期刊要求提供。
请看我们投稿后,编辑部在2017年的回复邮件中提出的意见,是不是很
“惊喜”
:
Title: ***
Dear Professor ***,
Please provide the name of the committee from which you received ethical approval for the use of rats in your study,
and provide evidence of this approval.
。。。
Yours sincerely,
***
2、
临床试验注册:
临床试验注册
”,大家也一定要注意,没有注册,估计后续会没有机会投稿。这个问题,似乎大家重视程度不够。
大家请看我们这两年遇到的一种情况,同时也有几个朋友反映:文章投稿后就拒稿,没有任何反馈意见。经过我们分析后,估计也是因为临床试验,没有伦理或者临床试验注册。
大家先请看要求提供临床试验注册的邮件(邮件是2015年编辑部回复的):
Dear Dr. **:
Before proceeding with the review orioles can you confirm the
clinical trial registry number
?
(
编辑部要求我们提供临床试验注册的注册号
)
. I look forward to hearing from you.
Sincerely,
Dr. **
Journal of ***
大家再来看看编辑部因为作者没有按照ICMJE规则要求来注册,没有提前注册而是后补注册的后果(这个来自2016年编辑部的
邮件
):
Dear Dr. **,
Many thanks for clarifying the status of your clinical trial registration. Unfortunately,
I cannot consider your manuscript further
.
As a journal, we adhere to the ICMJE guidelines, which require prospective trial registration(
ICMJE的要求,前瞻性研究的临床试验,必须在纳入第一例病人前完成临床试验注册
)
. In spite of the present decision, we look forward to submissions from you in the future.
Thank you for submitting your manuscript to the Journal of ***.
Yours sincerely
Dr. ***
然而,到这里,大家是不是认为:在还没有开始临床试验前,仅仅注册就可以了呢?
看看下面最新的要求吧,目前的要求是越来越高!
3、
ICMJE的新规:
临床试验注册的数据,必须全部公开!!!这是最新的要求,就在6月初公布的。大家请看:

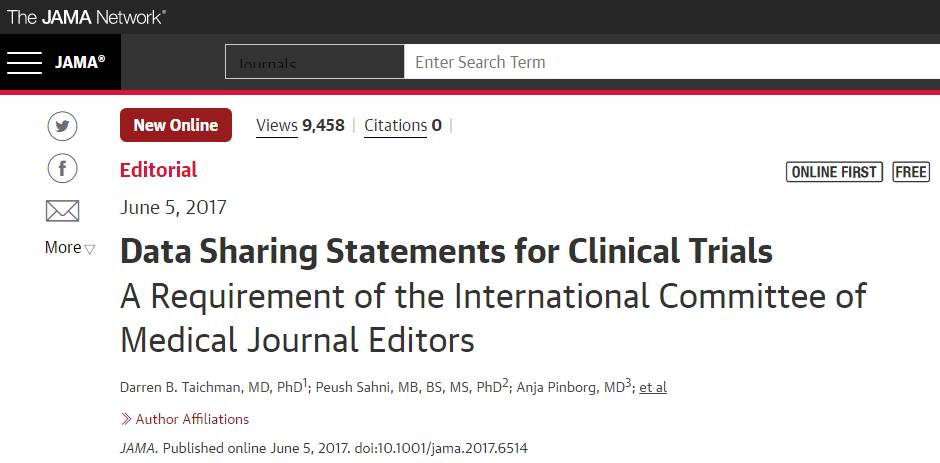
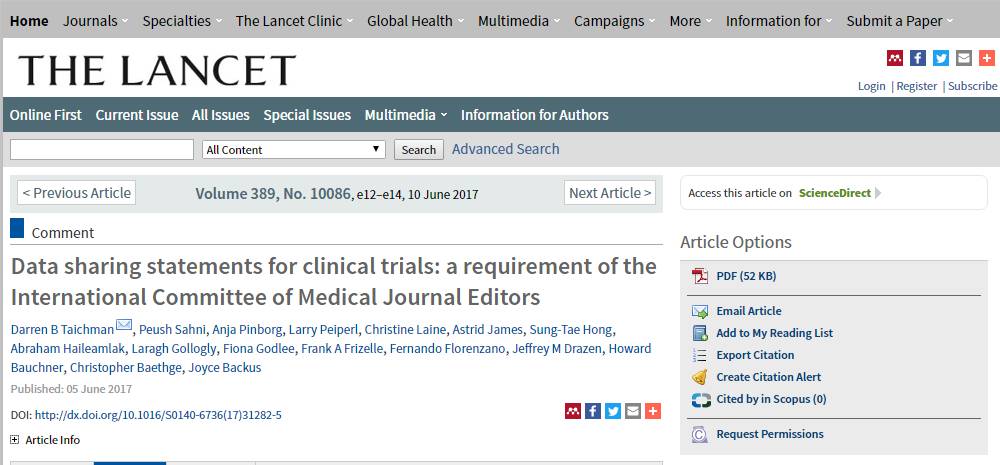
什么?什么?要全部公开?这是怎么回事??新规则下,医生还能不能好好发SCI论文了?
首先,我们看看ICMJE的要求是哪些?下图是ICMJE要求中提供的最新内容:
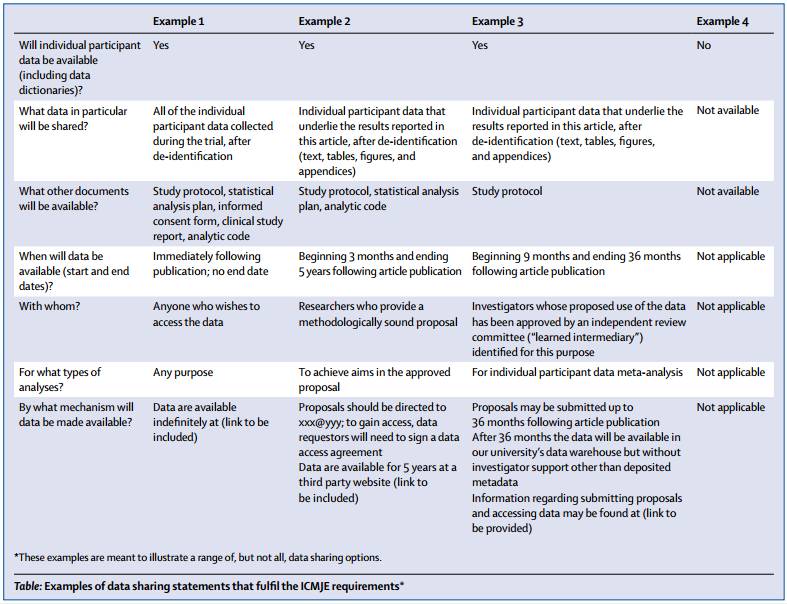
Therefore, ICMJE will require the following as conditions of consideration for publication of a clinical trial report in our member journals:
1. As of July 1, 2018, manuscripts submitted to ICMJE journals that report the results of clinical trials must contain a data sharing statement as described below.
2. Clinical trials that begin enrolling participants on or after January 1, 2019, must include a data sharing plan in the trial’s registration. The ICMJE’s policy regarding trial registration is explained at http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html. If the data sharing plan changes after registration this should be reflected in the statement submitted and published with the manuscript and updated in the registry record.
简单翻译一下,就是:
第一,2018年7月及以后提交到ICMJE期刊的临床试验报告,必须包含数据共享声明。
第二,2019年1月1日后开始入组受试者的临床试验,必须在临床试验注册平台上提交数据共享计划。
ICMJE为何这么牛气?我们来看看ICMJE的情况,ICMJE是International Committee of Medical Journal Editors(国际医学期刊编辑委员会)的简称。
ICMJE的成员包括哪些期刊呢?医学领域的话,我们搜索了一下ICMJE的网站(http://www.icmje.org/),得到如下信息: