本文整理自网络
同性生殖(unisexual reproduction)的现象在动物中并不罕见,如蜥蜴、蛙等;然而对于高等哺乳动物,无论孤雌生殖或孤雄生殖都不存在。
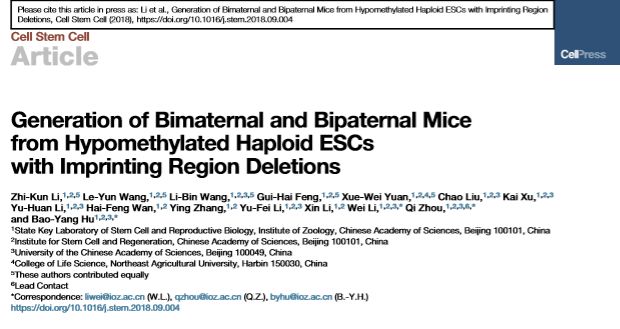
10月11日,中科院动物研究所胡宝洋研究员与周琪院士、李伟研究员组成的联合研究团队在Cell Stem Cell杂志上发表了题为Generation of Uniparental Mice from Hypomethylated Haploid ESCs with Imprinting Region Deletions的研究论文,结合单倍体干细胞技术和基因编辑技术对上述问题进行探索,
首次获得具有两个父系基因组的孤雄小鼠,具有十分重要的意义。
来自
Cell Stem Cell. 2018
后台回复“
孤雄小鼠
”,即可得全文下载!
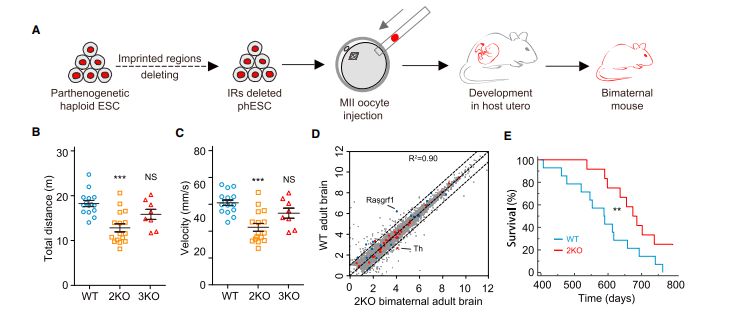
该研究团队首先发现,由卵细胞建立的孤雌单倍体干细胞,在高代次条件下,删除与Kono实验室相同的两个印记区段并注射进第二个卵细胞后,同样能发育得到“两个母亲”的孤雌小鼠。进一步研究发现,高代次的孤雌单倍体细胞展现出了一种不同于卵子或较低代次细胞,反而类似原始生殖细胞的全基因组甲基化模式,且所有的印记区段都呈现出类似原始生殖细胞的“无印记”状态。这揭示了为何 “仅仅删除2个”印记区段,就能获得孤雌小鼠:实际上,所有继承自卵细胞的印记基因,在孤雌单倍体干细胞中都经历了印记模式的“重新修正”。与此结果一致的是,研究人员发现在孤雌小鼠中,除了一个印记基因(Rasgrf1)表达显著异常之外,其他所有印记基因都与正常小鼠无异。
来自
Cell Stem Cell. 2018
研究人员在孤雄单倍体干细胞中,筛选并最终删除了7个重要的印记控制区段(Nespas,Grb10,Igf2r,Snrpn,Kcnq1,Peg3,Gnas)。这些细胞与另一颗精子所形成的孤雄胚胎干细胞,发育成为了活的孤雄小鼠。这些孤雄小鼠外观正常,可以自主呼吸,但是都在出生后48小时内死亡。
这是首次获得具有两个父系基因组的孤雄小鼠,证实了即便在最高等的哺乳动物中,孤雄生殖也有可能实现。
来自Cell Stem Cell. 2018
据悉,该研究工作由中国科学院动物研究所和中国科学院干细胞与再生医学创新研究院完成。中国科学院动物研究所胡宝洋研究员、周琪研究员和李伟研究员为论文的共同通讯作者。
以上整理自BioArtReports等
文献:
Li et al. Generation of Bimaternal and Bipaternal Mice from Hypomethylated Haploid ESCs with Imprinting Region Deletions. Cell Stem Cell. Published online: October 11, 2018
后台回复“
孤雄小鼠
”,即可得全文下载!
Highlights
• Haploid ESCs display PGC-like methylation profiles following in vitro cultivation
• Parthenogenetic and androgenetic haploid ESCs show different demethylation dynamics
• phESCs carrying 3 deleted imprinted regions support normal growth of bimaternal mice
• ahESCs carrying 7 deleted imprinted regions produce live full-term bipaternal mice
Summary
Unisexual reproduction is widespread among lower vertebrates, but not in mammals. Deletion of the H19 imprinted region in immature oocytes produced bimaternal mice with defective growth; however, bipaternal reproduction has not been previously achieved in mammals. We found that cultured parthenogenetic and androgenetic haploid embryonic stem cells (haESCs) display DNA hypomethylation resembling that of primordial germ cells. Through MII oocyte injection or sperm coinjection with hypomethylated haploid ESCs carrying specific imprinted region deletions, we obtained live bimaternal and bipaternal mice. Deletion of 3 imprinted regions in parthenogenetic haploid ESCs restored normal growth of fertile bimaternal mice, whereas deletion of 7 imprinted regions in androgenetic haploid ESCs enabled production of live bipaternal mice that died shortly after birth. Phenotypic analyses of organ and body size of these mice support the genetic conflict theory of genomic imprinting. Taken together, our results highlight the factors necessary for crossing same-sex reproduction barriers in mammals.
本文主要整理自BioArtReports等


如何选择SCI期刊,也是一门技巧。“
51选刊
”应此而建,目的主要是帮助大家选择拟投稿的SCI期刊!
“
51选刊
”目前已经开通了:
SCI期刊
自引率
、