HiMed:
我们的邻居(隔着金科路)再鼎医药今晚IPO,每天我都会从再鼎楼下走过~对杜总深感钦佩!VIC模式典范之作!在张江这边医药创新的热土上,我们会看到更多的再鼎,创业期的伙伴们加油!
转 | 药研发公众号
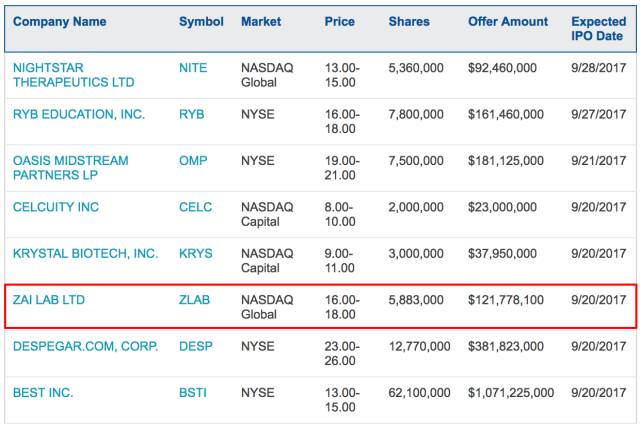
今晚再鼎医药将在纳斯达克正式IPO,股票交易代码"ZLAB"。承销商为摩根大通、花旗和Leerink。首次
公开募股计划募集资本1.22亿美元,
发行股份588.3万股(
设定发行价区间为16美元/股至18美元/股),
约为发行后总股本的12.53%

杜莹博士
2014年
1月创立再鼎医药。
2012年
加入红杉中国,任职红杉资本中国基金董事总经理。主导投资了贝达药业、百济神州、喜康寿生物(JHL)、华大基因和安琪儿医院。
2002年
创立和记黄埔医药上海有限公司并担任总裁,亦是和黄中国医药科技有限公司主要创立人及首席科技官,曾于2006年主持筹划其在英国上市。
1994年
加入美国辉瑞,曾在全球战略收购部工作,主管全球代谢类疾病项目转让及相关兼并收购。
再鼎股权结构

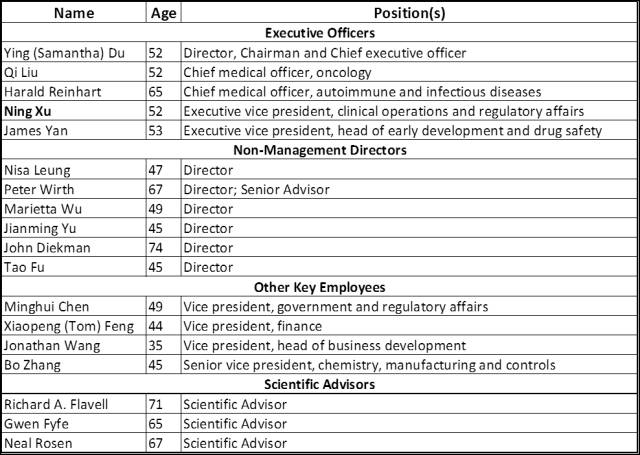
再鼎团队

Executive officers
Ying ( Samantha) Du
, Ph.D., co-founded our company and has been our director, chairman and chief executive officer since our inception. Prior to founding our company, Dr. Du spent two years as managing director of healthcare investments at Sequoia Capital China, where she led four investments. From 2001 to 2011, Dr. Du was founder and chief executive officer of Hutchison Medi-Pharma and the co-founder and chief scientific officer of Hutchison China MediTech Limited, a Nasdaq-listed biopharmaceutical company, where she pioneered China-based global biopharmaceutical innovation by bringing five internally-discovered innovative drug candidates into clinical trials, including two global Phase III ready drug candidates. Dr. Du began her career with Pfizer in the United States in 1994, where she was involved in the development and launch of two global drugs. While at Pfizer, she was responsible for Pfizer’s global metabolic licensing program on the scientific side. She received a Ph.D. in biochemistry from the University of Cincinnati. Dr. Du has also been involved with and chaired several Chinese regulatory and government related committees.
Qi Liu
, M.D., Ph.D. has been our chief medical officer, oncology since 2015. Prior to joining our company, Dr. Liu was the clinical head of the BioVenture group at AstraZeneca and the executive medical director of AstraZeneca Oncology, where she played an important role in establishing AstraZeneca’s biologics joint ventures and was responsible for its joint venture global clinical development programs and regulatory strategy and submissions. She also played a key role in the TKI development program. Prior to joining AstraZeneca, Dr. Liu was an assistant professor at the MD Anderson Cancer Center. Dr. Liu received a medical degree from Shanghai Medical University (currently known as Shanghai Medical College of Fudan University) and a Ph.D. in molecular genetics from the University of Georgia. She completed a post-doctoral fellowship at Memorial Sloan Kettering Cancer Center and a medical oncology and hematology fellowship at the MD Anderson Cancer Center with board certifications in internal medicine, medical oncology and hematology.
Harald Reinhart
, M.D., has been our chief medical officer, autoimmune and infectious diseases since 2017. He is currently adjunct clinical professor of infectious diseases at the Yale School of Medicine. Prior to joining our company, in 2012, Dr. Reinhart joined Shionogi US as head of Clinical Development and Medical Affairs, where he directed a broad portfolio of antibiotics, diabetes, allergy and pain medications, as well as guided a pharmaceutical product through NDA submission and approval. Between 2003 and 2011, Dr. Reinhart held senior roles at Novartis, where he oversaw successful filings of SNDAs and NDAs for Coartem, Famvir, Sebivo, and Cubicin, managed clinical development groups for transplantation, renal disease and immunity, and supervised the transitioning of projects from research into clinics. Dr. Reinhart received a medical degree from the University of Würzburg in Germany. He completed his medical specialty training in the United States with board-certifications in internal medicine and infectious diseases.
Ning Xu
, M.D., has been our executive vice president, clinical operations and regulatory affairs since 2014. Prior to joining our company, he served as vice president, head of clinical development service at Covance China. Before joining Covance, Dr. Xu served as a senior medical and regulatory affairs executive at Johnson & Johnson and GlaxoSmithKline. Dr. Xu received a medical degree from Peking Union Medical College and a master of business administration from the University of Illinois at Chicago. Dr. Xu also completed a postdoctoral fellowship at the Medical School, University of lllinois at Chicago. Between 2011 and 2015, he was the chairman of the Advisory Council of DIA China and a director of DIA Global.
James Yan
, Ph.D., has been our executive vice president and head of pre-clinical development and drug safety since 2015. Prior to joining our company, Dr. Yan was the head of the Covance early development Shanghai site, where he was responsible for all aspects of the business. Between 2009 and 2011, Dr. Yan served as the head of drug safety evaluation and program management of Hutchison Medi-Pharma. Prior to Hutchison Medi-Pharma, Dr. Yan had significant experience at Pfizer in the United States. Over the course of his career, Dr. Yan was been involved in many IND and NDA filings for multiple drug candidates and gained substantial experience working
再鼎研发产品线

















