Dr. Boarhead’s Summary of Global Updates on the 2019 Novel Coronavirus: 3 May 2021
1. 美国疾病控制与预防中心:
肯塔基州某护理院一名未接种疫苗的护理人员新冠检测呈阳性。当时该院 90% 的住院者与 53% 的护理人员已接种第二剂 BNT162b2 疫苗,且多数是在该名人员确诊前两周完成接种的。报告显示,该护理院 199 人中,46 人在本轮院内疫情中感染。研究人员估计,住院者中,BNT162b2 疫苗对有症状新冠肺炎的防御有效性为 86.5% ;护理人员中,有效性为 87.1% 。
( 4 月 21 日)
[关键信息]
一项研究表明,BNT162b2 疫苗的有效性超过 85% 。
US CDC:
An unvaccinated healthcare worker at a Kentucky care home tested positive for SARS-CoV-2. At that time, 90% of the home’s residents and 53% of its healthcare workers had received their second dose of the BNT162b2 vaccine; the vast majority had received their second shot more than 2 weeks before the worker’s infection was identified. It is reported that 46 of the 199 people at the care home were infected during the outbreak. The researchers estimate that the vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective among healthcare personnel.
<21 Apr.>
[key info]
The BNT162b2 vaccine is over 85% effective in a study.
[link]
https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e2.htm?s_cid=mm7017e2_w
2. 《新英格兰医学杂志》:
在一项对近四万人的试验中,Ad26.COV2.S 疫苗预防感染的有效性为 66% ,预防重至危重症新冠肺炎的有效性为 85% 。对于最早发现于南非的变异株,该疫苗预防中度新冠肺炎的有效性为 64% ,预防重至危重症新冠肺炎的有效性为 82% 。
( 4 月 21 日)
[关键信息]
Ad26.COV2.S 疫苗抵御南非变异株的有效性超过 60% 。
New England Journal of Medicine
:
In a trial involving nearly 40,000 persons, the efficacy of the Ad26.COV2.S vaccine was 66% against infection and 85% against severe to critical COVID-19. Efficacy against the variant first identified in South Africa was 64% against moderate disease and 82% against severe to critical disease.
<21 Apr.>
[key info]
Ad26.COV2.S is over 60% effective against the South Africa variant.
[link]
https://www.nejm.org/doi/full/10.1056/NEJMoa2101544?query=featured_coronavirus

3. 《自然》:
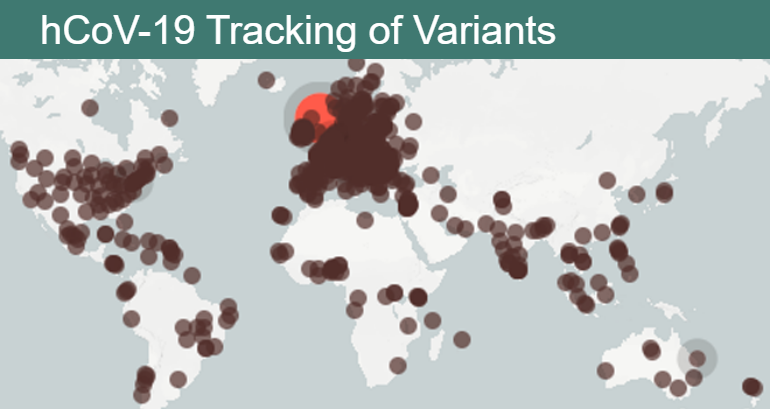
已有 172 个国家和地区在线上数据平台“禽流感数据分享全球倡议” GISAID 共享超过 120 万组冠状病毒基因组序列。该系统展示新冠病毒在世界范围内的传播方式,以及防控措施和疫苗的有效性。
( 4 月 23 日)
[关键信息]
GISAID 平台的冠状病毒基因组序列已超 120 万组。
Nature
:
More than 1.2 million coronavirus genome sequences from 172 countries and territories have now been shared on the online data platform GISAID, the Global Initiative on Sharing Avian Influenza Data. This system shows how SARS-CoV-2 spreads through the world and if control measures and the vaccines still work.
<23 Apr.>
[key info]
The number of coronavirus genome sequences on GISAID has surpassed 1.2 million.
[link]
https://www.nature.com/articles/d41586-021-01069-w
4. 美国国家公共广播电台:
在印度盛行的新型变异株中,有一种“双重变种”。其正式名称为 B.1.617 ,具有两处关键变异,已分别出现在若干其他变异株上。名为 L452R 的变异出现在美国加利福尼亚州主要流行的变异株上;名为 E484Q 的变异与南非、巴西首先发现的变异株上的变异类似。根据初步证据,B.1.617 的传染性比此前发现的变异株更强。
( 4 月 24 日)
[关键信息]
B.1.617 的传染性或比此前发现的变异株更强。
NPR:
One of the new variants circulating in India is referred to as the “double mutant.” Officially, the variant is called B.1.617, which has two key mutations that have cropped up in several other strains. The first mutation, labeled L452R, is also present in the dominant strain in California. The second one, called E484Q, is similar to the mutation in the variants detected first in South Africa and Brazil. Preliminary evidence suggests that B.1.617 is more contagious than previous variants.
<24 Apr.>
[key info]
The B.1.617 variant may be more contagious than previous variants.
[link]
https://www.npr.org/sections/goatsandsoda/2021/04/24/988744811/people-are-talking-about-a-double-mutant-variant-in-india-what-does-that-mean

5. 《柳叶刀》:
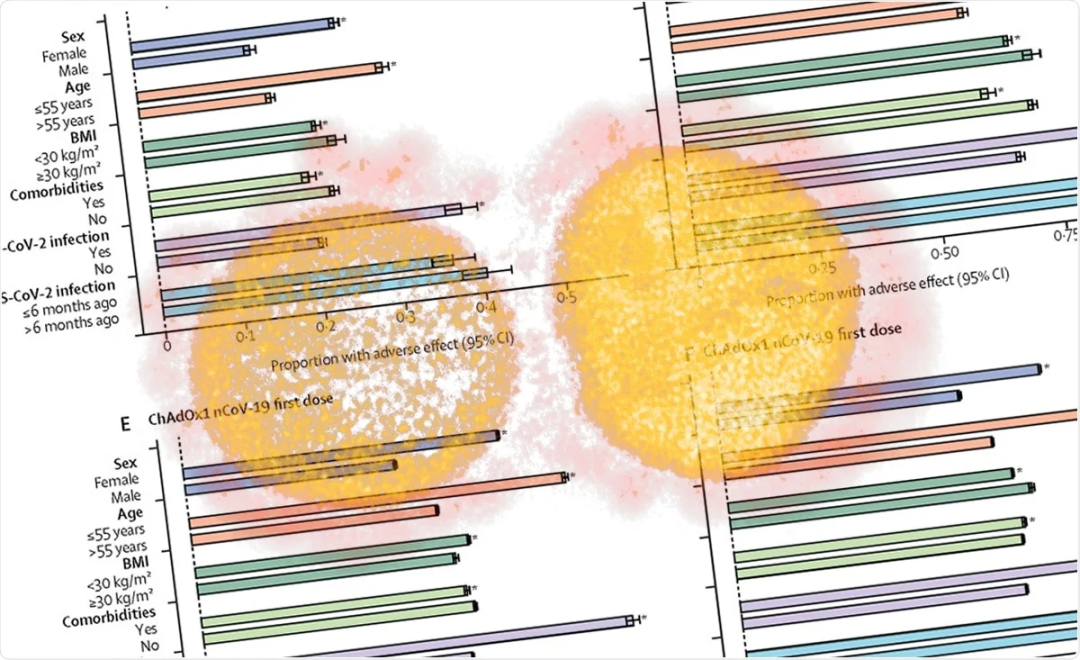
研究人员考察了“新冠肺炎症状研究”应用程序中疫苗接种八天内用户自主报告的全身和局部副作用的比例和概率。这些用户或接种一剂或两剂 BNT162b2 疫苗,或接种一剂 ChAdOx1 nCoV-19 疫苗。在这项研究中,副作用的频率低于 III 期试验报告结果。
( 4 月 27 日)
[关键信息]
自主报告的 BNT162b2 疫苗和 ChAdOx1 nCoV-19 疫苗接种副作用频率低于 III 期试验结果。
Lancet
:
Researchers examined the proportion and probability of self-reported systemic and local side-effects within 8 days of vaccination in individuals using the COVID Symptom Study app who received one or two doses of the BNT162b2 vaccine or one dose of the ChAdOx1 nCoV-19 vaccine. Side-effects in the study occur at frequencies lower than reported in phase 3 trials.
<27 Apr.>
[key info]
The frequencies of self-reported side-effects after BNT162b2 and ChAdOx1 nCoV-19 vaccinations are lower than phase 3 trial results.
[link]
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00224-3/fulltext
6. 《柳叶刀》:
单剂 BNT162b2 疫苗对癌症患者的预防作用不佳。实体癌患者如在接种首剂疫苗后第 21 天接种第二剂,则完成接种后两周内免疫原性显著增强。这些数据佐证,癌症患者宜优先尽早接种(第 21 天)第二剂 BNT162b2 疫苗。
( 4 月 27 日)
[关键信息]
癌症患者应优先接种第二剂疫苗。
Lancet
:
One dose of the BNT162b2 vaccine yields poor efficacy for patients with cancer. For patients with solid cancer, immunogenicity increased significantly within two weeks of a vaccine boost on day 21 after the first dose. These data support prioritization of patients with cancer for an early (day 21) second dose of the BNT162b2 vaccine.
<27 Apr.>
[key info]
Patients with cancer should be prioritized for the second dose of vaccine.
[link]
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(21)00213-8/fulltext






