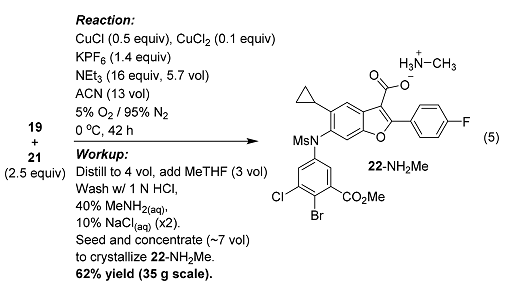
J
. Org. Chem. 2019, 84, 4680−4694. DOI:10.1021/acs.joc.8b02269
◆
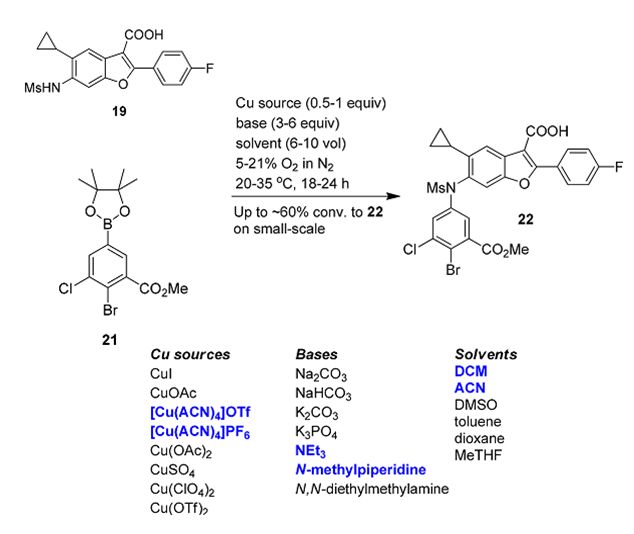
Cationic Cu(I) salts generally outperform other Cu(I) or Cu(II) sources in the Chan−Lam arylation of secondary sulfonamides. We attribute the superiority of cationic Cu(I) sources to the absenceof potentially competing X-type ligands (AcO−, Cl−, etc.) that could inhibit coordination of the sulfonamide.
◆
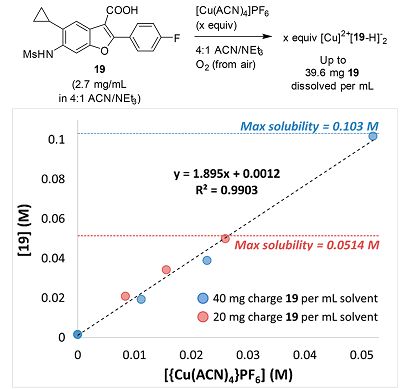
In acetonitrile, 19 still has poor solubility (2.9 mg/mL) when [Cu(ACN)4]PF6 is present; however, upon addition of triethylamine, the mixture clarifies and immediately turns dark green. We attribute this behavior to the oxidation of Cu(I) by air to generate a 2:1 complex between the anion of 19 and a Cu(II) center.
◆
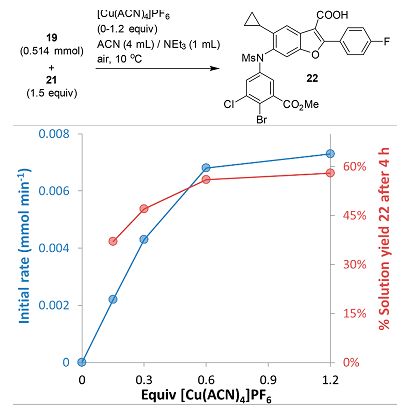
Given the correlation between the Cu loading and amount of dissolved 19, we also assessed the influence of Cu loading on the Chan−Lam reaction itself. While a detailed kinetic study is required to elucidate the reaction mechanism, it is clear that the optimal amount of Cu is 0.5−0.6 equiv.
◆
Small-scale experiments led to the identification of KPF6 in combination with CuCl as an effective replacement for [Cu(ACN)4]PF6. Unfortunately, this CuCl/KPF6 system failed to reach acceptable solution yields of 22 upon scale-up, where we suspected the poor solubility of CuCl leads to slow halide abstraction.
◆
Use of CuX2 (X = Cl, Br, I) instead of CuCl resulted in more consistent outcomes on multigram scales; however, we observed a significant new byproduct resulting from halogenation of the Ar−Bpin bond. Iterative optimization led us to a mixed CuCl/CuCl2 system in conjunction with KPF6, which performs well on scale without the need to premix the inorganics.