There has never been a more exciting pharmaceutical era in China, with the R&D emphasis shifting
from generic to innovative drugs. Oncology has been atthe forefront ofthis transition. Indeed,
12
out of
44
of the
new cancer drugs approved in China in between 2018 and August 2020
were
discovered in China. Although most were me-too drugs from established drug classes, first-in-class
candidates are also emerging.
Here, we overview the landscape of domestic oncology drug pipelines in China, with the aim of
providing insights on the evolution ofthe pharmaceutical ecosystem and R&D trends. For details
ofthe data and analysis as well as additional figures, see the Supplementary Information.
From me-too to first-in-class
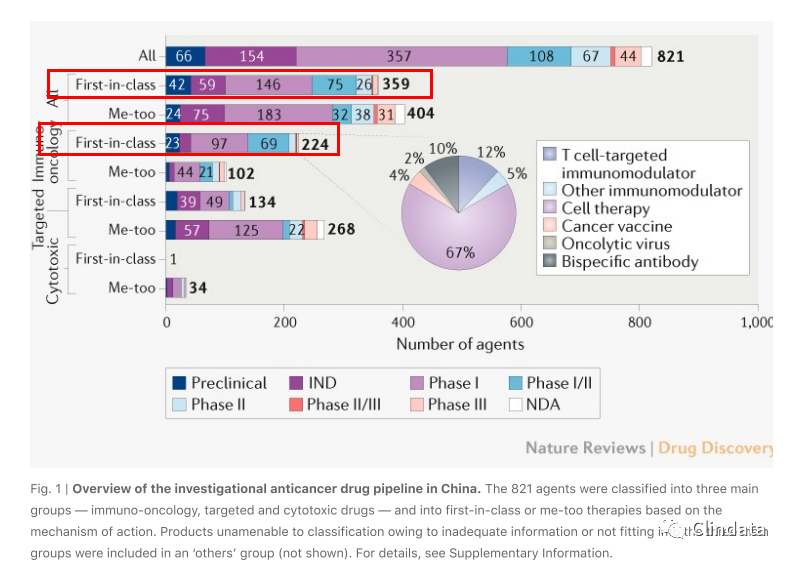
As ofJanuary 2020,there were 821 investigational anticancer drug candidates in China, including
404 me-too and 359 first-in-class agents (Fig. 1).
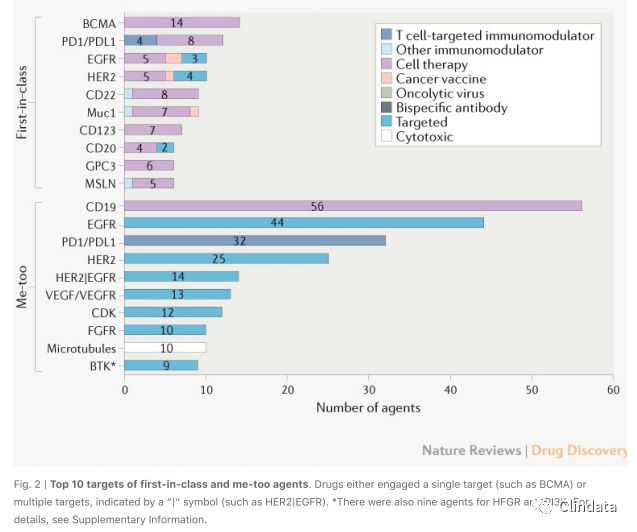
Fresh from the transition from generics, many start-up companies have opted for less risky and
potentially faster paths towards commercial viability by developing me-too drugs, in some cases
following closely behind the pioneering therapies such as celltherapies. The largest groups among
the me-too drugs were 56 CD19-directed chimeric antigen receptor (CAR) T celltherapies, 83
targeted therapies against EGFR or HER2 and 32 monoclonal antibodies targeting PD1 or PDL1 (Fig.
2).
Moderate market competition may increase availability and affordability for patients, but
developers who focus only on me-too drugs risk using resources less efficiently and hampering
long-term innovation. As the market becomes more established and self-regulated, limited market
shares in the context of a highly competitive environmentfor similar drugs may reduce the
attractiveness of me-too candidates. Aims of government policy, such as reduced profit margins
due to health insurance reforms and future requests by regulatory authorities to demonstrate
superiority in trials against active controls before marketing approvals may further promote the
development of drugs with best-in-class potential. In this respect,the EGFR inhibitor almonertinib
and the BTK inhibitor zanubrutinib are successful recent examples.
Cutting-edge scientific discoveries and technological revolution in China generally stem from
academia, as indicated by a higher proportion of first-in-class oncology agents developed by
academia than by industry (80% versus 40%; Supplementary Fig. 1). Further translation into
products and commercialization requires participation from industry. The pioneering
development of glypican 3 (GPC3)-directed CAR T cells (GPC3-CART) provides one illustration of
collaboration between academia and industry.
Looking atthe first-in-class pipeline,there are a substantial number of candidates in the immunooncology (IO) field. Celltherapies are particularly prominent, making up 150 out ofthe 224 first-inclass IO agents (67%, Fig. 1, Supplementary Fig. 2). Celltherapies also dominate the top 10 targets
for first-in-class agents, such as BCMA, CD22, mucin 1, CD123 and GPC3 (Fig. 2; Supplementary
Table 1). This level ofinnovation is reflected in China’s position in the global celltherapy pipeline,
second only to the United States in terms ofthe number of agents in development(Nat. Rev. Drug
Discov. 19, 583–584; 2020). This has been stimulated by the government’s prioritization and
regulatory reforms. Since December 2017, China has reconfigured the landscape for celltherapy
development, by incorporating itinto the Thirteenth Five-Year Plan and issuing technical
guidance.
Newer technologies are also offering possibilities of pursuing established single or multiple targets
in a novel way (Fig. 2; Supplementary Fig. 3). Examples include celltherapies targeting PD1/PDL1,
EGFR and HER2, HER2-based bispecific antibodies and CD19-based bispecific celltherapies. Multitargeted drugs may offer transformative potential, given that combination treatmentis already a
cornerstone of cancer therapy.
Unmet needs drive innovation
In the overall pipeline, 495 products are being developed for solid tumours, with targeted
therapies making up the largest group (62%, Supplementary Fig. 4). Despite the major challenges
in tackling solid tumours with celltherapies,there are also 83 celltherapies for such tumours in
development. Celltherapies make up around half(51%) ofthe 249 products being developed for
haematologicaltumours. Frequenttargets include the established target CD19, as well as the firstin-class targets CD22 and BCMA.
Using disability-adjusted life years (DALYs), a measure of disease burden incorporating morbidity,
mortality and disability,to approximately reflect unmet medical needs, our analysis indicated a
strong correlation between the number of drugs and the corresponding disease burden in China
across 23 solid tumours and 7 haematological cancers (Supplementary Fig. 5). With the highest
DALY burden in China, lung cancer had the greatest number of candidate drugs,the majority of
which were me-too drugs. For some cancers with a high DALY burden in China, such as esophageal,
gastric and hepatic cancer,the numbers of candidate drugs were lower than might be expected on
the basis of a linear correlation, butthe proportions that were first-in-class were relatively higher
(Supplementary Fig. 4 and 5), atleast partially owing to the lack of validated targets for these
cancers.
Innovation in and out
China has now become a major target country for R&D globalization. One way to penetrate China
for overseas developers, especially biotech companies, is through in-licensing to Chinese
biopharma companies. In early 2020,the proportion ofin-licensed candidates in the overall
pipeline rose to 18% (147),transferred primarily in preclinical or early clinical phases (Fig. 3).
Typical development paths initially focused on niche indications or advanced tumours, which may
shorten the time to reach the Chinese market. For example, niraparib was licensed to Zai Lab by
Tesaro in 2016 and granted marketing approvalfor recurrent ovarian cancer in China in 2019.
Notably, 30 investigational products were at concurrent stages in and outside China (Fig. 3),
highlighting China’s openness towards accepting foreign data.
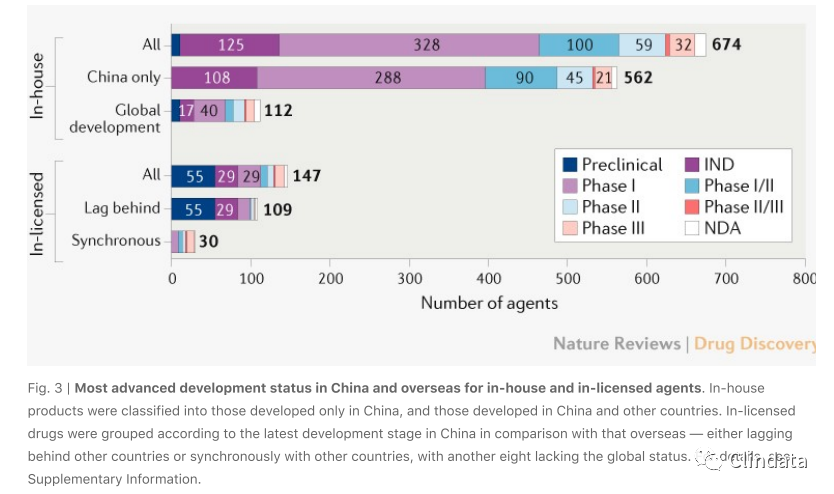
Products discovered in China are also being developed outside the country (112 out of 674 agents;
(Fig. 3)), indicating the external recognition of Chinese discovery capabilities, manufacturing
quality and study design. In particular, 17 agents were out-licensed to companies externally. For
example,the BCMA-directed CAR T celltherapy LCAR-B38M, which has received breakthrough
therapy designation in the United States and China, was licensed by Janssen Biotech from Legend
Biotech in 2017.
Outlook
Although the oncology pipeline in China is being driven by the unmet medical needs,the
innovation could be compromised by inappropriate trial design and excessive expenditure but
insufficient rewards. Future oncology trials will need to adaptto innovative, integrative platforms
to test multiple candidates in designated patient populations using resource-optimized and costefficient approaches. If China continues to develop an environmentthat encourages innovation
and the regulatory agency keeps an open mind about noveltechnologies and products,the
emergence of a cluster of home-grown, ground-breaking therapies can be anticipated,through
collaboration between academia and industry in China and beyond.





