这周三,我们一起来看一篇发表在
Cancer Science (IF:3.896)
上的文章——
Wnt5b-associated exosomes promote cancer cell migration and proliferation
摘要:
Wnt5b
和
Wnt5a
一样同属一个蛋白质家族,它的过度表达与癌症的侵袭密切相关。
Wnt5b
也被认为与癌症的进展有关,但其机制并不清楚。我们在癌细胞中分析了纯化的
Wnt5b
的生化特性以及它的分泌模式。
Wnt5b
在三个天冬酰胺残基上被糖化,在一个丝氨酸残基上被脂化,这些转录后修饰是
Wnt5b
分泌所必须的。纯化的
Wnt5b
有着和
Wnt5a
相似的
Dvl2
磷酸化和
Rac
激活能力。细胞培养基经
100000g
离心后,能够在上清和沉淀中发现
Wnt5b
。在胰腺癌细胞
PANC-1
中,分泌的
Wnt5b
,
55%
与外泌体有关。从野生型
PANC-1
细胞中分离得到的外泌体,能够激活
CHO
细胞的
Wnt5b
信号通路并且能够促进肺腺癌细胞
A549
的迁移和增殖,因此认为外泌体相关的内源性
Wnt5b
是非常活跃的。外泌体从
CHO
细胞中获得,并通过免疫电镜验证,
Wnt5b
确实来自外泌体。在结肠癌细胞
Caco-2
中,当
Wnt5b
异位表达时
(Caco-2/Wnt5b cells)
,绝大多数的
Wnt5b
在沉淀组分中被发现。敲低
TSG101
,一种外泌体的标志物,降低了
Caco-2/Wnt5b
细胞
Wnt5b
相关外泌体的分泌,并抑制了
Wnt5b
依赖性细胞的增殖。
Caco-2/Wnt5b
细胞分泌的外泌体能刺激
A549
细胞的迁移和增殖。以上结果表明
Wnt5b
相关外泌体以一种旁分泌的方式来促进癌细胞的迁移和增殖。
Fig. 1. Determinationof the glycan profiles of Wnt5b. (a) Coomassie Brilliant Blue (CBB) staining ofan SDS polyacrylamide gel containing fractions from all chromatographies in thepurification process. CM, conditioned medium. (b) Purified Wnt5a and Wnt5bproteins were stained with CBB and probed with anti-Wnt5a and anti-Wnt5b antibodies.(c) Wnt5b glycopeptides obtained from nano-flow liquid chromatography wereseparately analyzed by MALDI mass spectrometry (MS). (d)MALDI mass spectrum of apeptide containing Ser223 of Wnt5b. (e) MALDI-MS/MS spectrum from the ion atm/z 1455.9 in (d). The sequences from the N- and C-termini and the position ofthe modified residue were read out based on y’’l and bm ions.

Fig. 2. Wntsecretion modes vary among cell lines.(a) Conditioned medium (CM) of L cellsstably expressing Wnts was separated into 100 000 g precipitates (P100) andsupernatants (Sup). Each fraction was probed with anti-Wnt5a/b or antiWnt3aantibodies. Wnt signals were quantified using ImageJ (National Institutes ofHealth, Bethesda, MD, USA) and results expressed as the ratio of Wnts in P100and Sup fractions. The sum of Wnts secreted from cultured cells into P100 and Supfractions was set to 100%. Results are shown as means SD of three independent experiments. (b) P100and Sup prepared from HeLaS3, A549, and KKLS cells were probed withanti-Wnt5a/b antibody. Arrowheads indicate the position of Wnt5a. (c) P100 andSup from Caco-2 cells transiently expressing indicated Wnts were probed withanti-Wnt5a/b or anti-Wnt3a antibodies. (d) P100 and Sup prepared from PANC-1cells were probed with the indicated antibodies. (e) P100 prepared from PANC-1 cellswas subjected to discontinuous sucrose gradient analysis, and samples offractions were probed with the indicated antibodies.

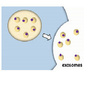
Fig. 3.Development of reporter assay to measure Wnt5a and Wnt5b signaling. (a) Schemaof the reporter system used to measure Wnt5a/b signaling using CHO cells stablyexpressing a Ror2– low-density lipoprotein receptor-related protein 6 (LRP6)chimera and TOP-flash (CHO/Ror2-LRP6-TOP cells). GSK3-b, glycogen synthasekinase 3b; TCF/LEF, T-cell factor/lymphoid enhancer factor. (b)CHO/Ror2-LRP6-TOP cells were stimulated with 1 Ml control, Wnt3a, or Wnt5aconditioned medium (CM) for 3 h, and cell lysates were probed withanti-phospho-LRP6 and anti-LRP6 antibodies. (c) CHO/Ror2-LRP6-TOP cells andparental CHO cells stably expressing only TOP-flash were stimulated withcontrol or Wnt5a CM for 8 h. A luciferase assay was carried out and theactivity was expressed as a fold increase compared with samples without Wnt5aand Ror2-LRP6. Results are shown as means
±
SD of three independent experiments. *P
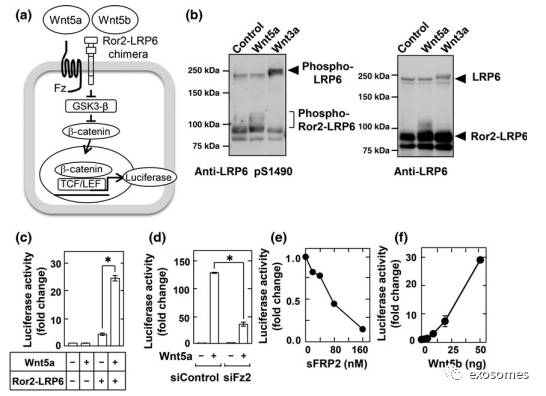
Fig. 4. Wnt5b-associatedexosomes from PANC-1 cells are active. (a) Schematic drawing of the targetingsite of single guide RNA (sgRNA) at exon 3 of human WNT5B gene, and thenucleotide sequences of both alleles from wild-type (WT) and WNT5B knockout(KO) PANC-1 cells are shown. A dash represents a single base deletion. (b)Lysates from WT and WNT5B KO cells were probed with anti-Wnt5b antibody. Hsp90,heat shock protein 90. (c) WT and WNT5B KO PANC-1 cells were subjected tomigration and invasion assays and relative migration and invasion activities ofWNT5B KO PANC-1 cells were expressed as percentages of those of wild-type PANC-1cells. Results are shown as means
±
SD of three independent experiments.*P
Fig. 5. Wnt5b-associatedexosomes are taken up by CHO cells. (a, b) CHO cells were incubated with PKH26-labeledPANC-1 exosomes for the indicated times at 4°C or 37°C. Representative images(a) and quantification of exosome uptake (b) are shown. Scale bar = 20 lm. (c)CHO cells incubated with PKH26-labeled PANC-1 exosomes were stained with phalloidin(F-actin, green) and DRAQ5 DNA Dye (nucleus, blue). Upper panel, verticalsection of the CHO cells (0.3-lm steps); lower panel, side views of the CHOcells are merges of 35 vertical sections of confocal stacks. Scale bar = 10 lm.(d) CHO/Ror2-LRP6-TOP cells were transfected with Wnt5b or HAWnt5b for 48 h. Aluciferase assay was carried out and the activity was expressed as a fold increasecompared with mock-transfected cells. (e) Left, lysates from mock and HA-Wnt5btransfected PANC-1 cells were probed with anti-HA antibody. Right, The 100 000g precipitates (P100) and supernatants (Sup) from PANC-1 cells transiently expressingHA-Wnt5b were probed with anti-HA antibody. (f) Exosomes isolated from PANC-1cells transiently expressing HA-Wnt5b were immunolabeled using anti-HA andanti-TSG101 antibodies followed by gold-conjugated secondary antibodies and thesamples were observed by electron microscopy. HA-Wnt5b, 25-nm gold particle;TSG101, 10-nm gold particle. Scale bar = 100 nm.

Fig. 6. Wnt5b-assocatedexosomes from Caco-2 cells are active. (a) Caco-2 cells stably expressing GFPor Wnt5b were transfected with the indicated siRNAs and subjected to cellproliferation assay. Results are shown as mean
±
SD of three independent experiments. *P