近日,ICH发布了Q3C(R8)的草案,中英文对照版如下:
ICHHARMONISED GUIDELINE
ICH 协调指南
IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS
杂质:残留溶剂指南
Q3C(R8)
PDE FOR 2-METHYLTETRAHYDROFURAN (2-MTHF), CYCLOPENTYL METHYL ETHER (CPME),
AND TERTIARY BUTYL ALCOHOL (TBA)
2-
甲基四氢呋喃(
2-MTHF
),环戊基甲醚(
CPME
)和叔丁醇(
TBA
)的
PDE
值
2-METHYLTETRAHYDROFURAN
2-
甲基四氢呋喃
Introduction
概述
2-Methyltetrahydrofuran (2-MTHF, synonyms: 2-Methyloxolane, Tetrahydrosylvan; Tetrahydro-2-methylfuran; CAS Number 96-47-9) is a colourless, volatile liquid with ether-like odour. 2-MTHF is an organic solvent usually synthesized as a racemic mixture consisting of two enantiomeric forms ((S)+ and (R)-). Solubility in water is limited and decreases with increasing temperature. It has a vapour pressure of 136 mbar (20°C) (1).
2-
甲基四氢呋喃(
2-MTHF
,别名
A-
甲基四氢呋喃、四氢
-2-
甲基呋喃,
CAS
号
96-47-9
),无色,挥发性液体,有醚类气味。
2-MTHF
常用于具有对映形态(
S
和
R
)手性混合物合成。其在水中溶解性有限,并随温度升高而降低。其蒸汽压为
136 mbar
(
20°C
)。
2-MTHF is increasingly used as a catalytic solvent in exchange of Tetrahydrofuran (THF) and is much less miscible with water compared to THF.
由于
2-MTHF
比
THF
更不易溶于水,目前
2-MTHF
被越来越多地用于催化反应的溶剂来取代
THF
。
Genotoxicity
基因毒性
2-MTHF was not mutagenic in the AMES bacterial reverse mutation assay with Salmonella typhimurium (3) and Escherichia coli WP2 uvrA (2). 2-MTHF was also tested in vitro in a L5178Y mouse lymphoma cell TK+/- assay (MLA) (3), and a chromosome aberration assay in human peripheral blood lymphocytes (2), and in vivo in a bone marrow micronucleus test integrated into a 3-month oral repeated-dose toxicity study in rats (2). All test results were negative except for the MLA in the presence of S9, which was considered inconclusive without further explanation (3). In conclusion, there is no evidence that 2-MTHF is genotoxic.
使用鼠伤寒沙门菌和大肠埃希氏杆菌
WP2 UVRA
进行的
AMES
细菌可逆致突变实验显示
2-MTHF
无致突变性。
2-MTHF
亦进行了体外
L5178Y
小鼠淋巴癌细胞
TK+/-
测试
(MLA)
,人体周边血液淋巴球染色体畸变试验,以及大鼠
3
个月经口重复给药毒性研究中的体内骨髓细胞微核试验。所有试验结果均为阴性,只有存在
S9
时有
MLA
,无进一步解释,可认为是未得出结论。总之,无证据证明
2-MTHF
具有基因毒性。
Carcinogenicity
致癌性
No data for 2-MTHF are available.
2-MTHF
尚无致癌性数据。
Reproductive toxicity
生殖毒性
No reliable information about reproductive toxicity is available. In an acute embryo toxicity and teratogenicity test in zebrafish, 2-MTHF was tested at concentrations ranging from 860 –8600 mg/L (4). Acute embryo toxicity was observed for 2-MTHF at a nominal LC50 value of 2980 mg/L. Sublethal effects were also observed, such as an increase in oedema at nominal concentrations ≥ 1720 mg/L, as well as an increased number of embryos without detectable blood circulation and insufficient pigmentation at a nominal concentration of 2580 mg/L.
无可靠生殖毒性信息。在一项斑马鱼急性胚胎毒性和致畸胎性测试中,使用浓度为
860 –8600 mg/L
的
2-MTHF
进行了试验,发现其急性胚胎毒性名义
LC50
值为
2980mg/L
。同时观察到亚致死效果,如名义浓度
≥ 1720 mg/L
时水肿增加,名义浓度为
2580 mg/L
时胚胎数量增加,未发现血液循环和色素沉着不足。
Teratogenic effects were not observed with 2-MTHF in this assay.
该项试验中未观察到
2-MTHF
胚胎受影响。
Repeated-dose toxicity
重复给药毒性
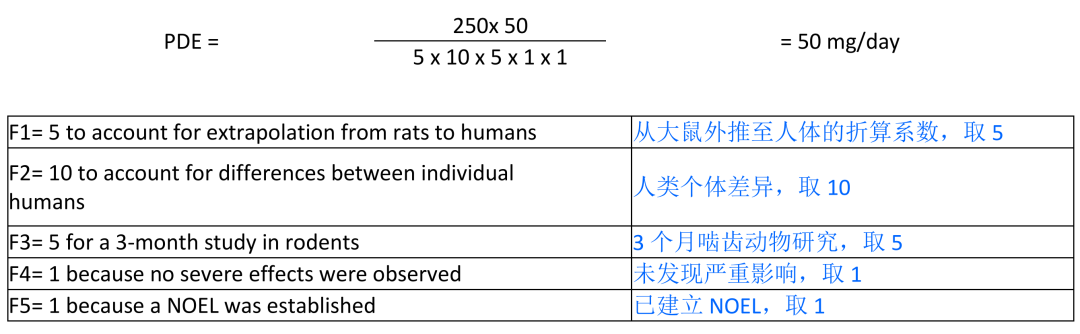
Two 3-month oral repeated-dose toxicity studies in Crl:CD (SD) rats have been described with 2-MTHF; one without an additional recovery period (2) and one with an additional 1-month recovery period (5). The top dose in the first study was 26 mg/kg/day (2) and in the second study 1000 mg/kg/day (5). 2-MTHF treatment-related observations were not seen in the first study (2). In the second study, groups of 10 male and 10 female rats per dose group were treated with doses of 80, 250, 500 and 1000 mg/kg/day (5). An additional 1-month treatment-free recovery period was added for 5 animals/sex of the control and the high dose groups. Treatment-related observations were generally seen only at doses ≥ 500 mg/kg/day. Besides slight effects on kidney weights (increased at ≥ 500 mg/kg/day), blood cholesterol (increase at 1000 mg/kg/day) and prothrombin time (decreased at ≥ 500 mg/kg/day), the only test article-related microscopic observation was hepatocellular centrilobular hypertrophy at 1000 mg/kg/day. However, no effects were observed in the recovery group and the observed effects can therefore be regarded as completely reversible (5). The NOEL in the second study was considered to be 250 mg/kg/day.
2
起使用
Crl:CD (SD)
大鼠的
3
个月口服重复给药毒性研究,一个没有增加恢复期,一个增加了
1
个月恢复期。第一起研究中给药剂量最高为
26mg/kg/
天,第二起研究中为
1000mg/kg/
天。在第一起研究中未观察到
2-MTHF
治疗结果。在第二起研究中,每个给药剂量含
10
只雄性和
10
只雌性大鼠,分别采用
80
、
250
、
500
和
1000mg/kg/
天剂量,并对其中
5
对大鼠增加
1
个月无治疗恢复期。作为高剂量组控制。仅在剂量
≥ 500 mg/kg/
天的试验组中发现治疗相关现象。除了对肾重量(增重
≥500mg/kg/
天)、血胆固醇水平(增加
1000 mg/kg/
天)和凝血酶原时间(降低
≥ 500 mg/kg/
天)有轻微影响外,仅在研究材料相关显微观察中发现肝细胞小叶中心肥大(
1000 mg/kg/
天)。但恢复组中未发现影响,因此所发现的影响可认为是完全可逆的。第二起研究中的
NOEL
为
250mg/kg/
天。
The NOEL of 250 mg/kg/day was used in the PDE calculation:
PDE
计算中采用
NOEL
值
250 mg/kg/
天。
Conclusion
结论
The calculated PDE for 2-MTHF is 50 mg/day based upon the NOEL of the rat sub-chronic oral study. Since the proposed PDE is greater than or equal to 50 mg/day, it is recommended that 2-MTHF be placed into Class 3 “Solvents with low toxic potential” in Table 3 in the ICH Impurities: Residual Solvents Guideline.
根据上述大鼠亚长期口服研究结果,
2-MTHF
的
PDE
计算结果为
50mg/
天。由于所得
PDE
值大于或等于
50mg/
天,因此建议将
2-MTHF
归为
ICH
杂质:残留溶剂指南表
3
中的第
3
类溶剂
“
毒性较低的溶剂
”
。
References
参考文献
Aycock DF. Solvent applications of 2-methyltetrahydrofuran in organometallic and biphasic reactions. Org. Process Res. Dev. 2007;11:156-159.
有机催化和双相反应中
2-MTHF
溶剂的应用
Antonucci V, Coleman J, Ferry JB, Johnson N, Mathe M, Scott JP, et al. Toxicological assessment of 2-methyltetrahydrofuran and cyclopentyl methyl ether in support of their use in pharmaceutical chemical process development. Org. Process Res. Dev. 66 2011;15:939-41.
支持
2-MTHF
和
CPME
在药物化学工艺开发中的毒性评估
Seifried HE, Seifried RM, Clarke JJ, Junghans TB, Sanet RH. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol 2006;19(5):627-44.
20
年采用
AMES
鼠伤寒沙门菌和
L5178Y
小鼠淋巴瘤细胞突变试验的致癌性研究结果汇编
Bluhm K, Seiler TB, Anders N, Klankermayer J, Schaeffer A, Hollert H. Acute embryo toxicity and teratogenicity of three potential biofuels also used as flavor or solvent. Sci 72 Total Environ. 2016;566-7:786-95.
亦可用作香料或溶剂的
3
种潜在生物燃料的急性胚胎毒性和致畸胎性
Parris P, Duncan JN, Fleetwood A, Beierschmitt WP. Calculation of a permitted daily exposure value for the solvent 2-methyltetrahydrofuran. Regul Toxicol Pharmacol 75 2017;87:54-63.
溶剂
2-MTHF
的允许日暴露值计算
CYCLOPENTYL METHYL ETHER
环戊基甲醚(
CPME
)
Introduction
概述
Cyclopentyl methyl ether (CPME: CAS Number 5614-37-9) is used in pharmaceutical chemical development as an alternative to its more common analogues such as tetrahydrofuran and tert-butyl methyl ether (1,2).
环戊基甲醚(
CPME
,
CAS
号
5614-37-9
)被用于药物化学开发中,替代其类似物,如四氢呋喃和叔丁基甲基醚(甲基叔丁基醚)。
The vapour pressure of CPME is 44.9 mmHg at 25°C, the Log Pow is 1.59 and the water 84 solubility is 1.1 g/100 g (23 °C) (3,4).
CPME
蒸汽压为
44.9mmHg
(
25°C
),
Log Pow
为
1.59
,水中溶解度为
1.1 g/100 g (23 °C)
。
CPME is classified as an irritant to skin (H315) and eye (H319) in accordance with EC No 1272/2008, in the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). CPME did not show the potential to induce skin sensitization in the Local Lymph Node Assay. In rats, LD50 for acute oral exposure is 1000–2000 mg/kg, for dermal exposure it is greater than 2000 mg/kg, and for inhalation exposure it is greater than 21.5 mg/L. No human toxicity data have been reported (2).
根据
EC 1272/2008
,
CPME
在全球化学品统一分类标签体系(
GHS
)中被归为
H315
(对皮肤无刺激)和
H319
(对眼睛无刺激)。
CPME
在局部淋巴结试验(
LLNA
)中未显示有诱发皮肤敏感的可能。大鼠试验中,急性口服暴露
LD50
值为
1000–2000 mg/kg
,皮肤暴露
LD50
值大于
2000 mg/kg
,吸入暴露
LD50
大于
21.5 mg/L
。无人体毒性数据报告。
Genotoxicity
基因毒性
The results of genotoxicity tests have been reported (1,2). CPME was not mutagenic genotoxic in the AMES bacterial reverse mutation assays in S. typhimurium test strains TA98, TA100, TA1535, TA1537 and E. coli WP2 uvrA with and without metabolic activation at concentrations up to 5710 µg/plate (1) and 5000 µg/plate (2). Negative results were also obtained in in vitro mammalian chromosome aberration tests in human lymphocytes at concentrations up to 1.1 mg/mL and in Chinese Hamster Lung cells at concentrations up to 1.0 mg/mL (2). An in vivo rat micronucleus test integrated in a 3-month oral repeated-dose study up to a dose of 31 mg/kg/day (1) and an in vivo mammalian erythrocyte micronucleus test in CD-1 mice at single oral doses up to 2000 mg/kg/ (2) also did not indicate any genotoxic potential. In conclusion, there is no evidence that CPME is genotoxic.
未见基因毒性试验结果报道。在鼠伤寒沙门氏试验菌
TA100, TA1535, TA1537
和大肠杆菌
WP2 uvrA
,浓度为
5710 µg/
碟和
5000 µg/
碟,有代谢活化和无代谢活化的
AMES
细菌反向突变试验中,
CPME
未显示诱变毒性。在浓度达
1.1mg/ml
的人类淋巴球的哺乳动物染色体畸变中,和
1.0 mg/mL
中国仓鼠肺细胞(
CHL
)体外试验中,未发现不良结果。给药剂量为
31mg/kg/
天的
3
个月口服重复给药研究里包括的体内大鼠微核试验中,和单一口服剂量为
2000mg/kg/
天
CD-1
小鼠体内哺乳动物红细胞微核试验中,亦未显示任何基因毒性可能。总之,无证据证明
CPME
具有基因毒性。
Carcinogenicity
致癌性
No data are available.
无数据
Reproductive toxicity
生殖毒性
In a two-generation reproductive toxicity study, CPME was administered to rats in drinking water at doses of 313, 1250 or 5000 mg/mL (5). Other than decreased body weights of pups in the F1 generation and F2 generation which were observed at the highest dose, no other significant changes in reproductive parameters were reported. The NOAEL of this study was estimated to be 193.45 mg/kg/day (1250 mg/L in drinking water). However, as detailed toxicity information from this study is not available, this study was not used to support the calculation of a PDE.
1
起
2
代生殖毒性研究中,采用饮水给大鼠服用
CPME
,给药剂量为
313
、
1250
和
5000 mg/mL
。除
F1
代和
F2
代高剂量给药大鼠幼体中发现体重降低外,未报道其它生殖参数重大变化。该研究估算
NOAEL
为
193.45 mg/kg/
天(饮水中浓度
1250 mg/L
)。但是该研究的详细毒性信息无法获得,未使用该研究支持
PDE
的计算。
Repeated-dose toxicity
重复给药毒性
CPME was studied in two oral and one inhalation repeated-dose studies in rats.
CPME
有
2
起大鼠口服重复给药和
1
起吸入重复给药研究。
In a 28-day study with a 14-day recovery period, Crj: Crl:CD(SD) rats were administered CPME by oral gavage at 15, 150 or 700 mg/kg/day in corn oil (2,6). Six unscheduled deaths occurred in males at 700 mg/kg/day between days 12 and 15 of treatment and were attributed to poor clinical conditions. Salivation was commonly observed in males and females at 700 mg/kg/day. Salivation occurred twice in one male at 150 mg/kg/day however this finding was not considered adverse. Decreased motor activity, piloerection, abnormal gait, tremors, convulsion, hunched posture, fast respiration, and thin appearance were observed in males at 700 mg/kg/day. Decreased body weight gain was observed in females at 700 mg/kg/day. All clinical findings and changes in bodyweight gains resolved after the recovery period. There were no other toxicological effects of CPME in this study. The NOEL of this study was determined to be 150 mg/kg/day.
在一项有
14
天恢复期的
28
天研究中,
Crj: Crl:CD(SD)
大鼠
CPME
口服管饲采用玉米油浓度分别为
15
、
150
和
700 mg/kg/
天。给药浓度为
700 mg/kg/
天的雄性大鼠在
12
和
15
天治疗时有
6
只计划外死亡,其原因归为不良临床条件。雄鼠和雌鼠在
700mg/kg/
天给药量时均发现有流涎症状。
150mg/kg/
天给药组有一只雄鼠观察到有流涎症状,但该发现并未作为是不良反应。
700mg/kg/
天给药组观察到运动活性降低、立毛、步态异常、战栗、痉挛、弓背姿势、呼吸急促和外貌差。
700mg/kg/
天给药组观察到体重增加降低。所有临床观察结果和体重增加的变化在恢复期得到解决。在此研究中未发现
CPME
的其它毒性影响。该研究确定
NOEL
为
150mg/kg/
天。
In a 90-day study, Sprague Dawley Crl:CD(SD) rats were administered up to 31 mg/kg/day CPME by oral gavage in corn oil (1). There were no CPME-related ante-mortem or post- mortem findings. Detailed information on the experimental design and study results such as clinical signs, haematology and blood chemistry findings were not publicly available, although the authors considered the NOEL of this study to be 31 mg/kg/day.
在一起
90
天研究中,斯泼累格
·
多雷
Crl:CD(SD)
大鼠采用口服玉米油中饲喂给
CPME 31mg/kg/
天。死前或死后未观察到
CPME
相关问题。该研究的设计和研究结果如临床信号、血象和血液化学观察结果详细信息无法通过公开途径获得,该作者从该研究得出
NOEL
为
31 mg/kg/
天。
In a 90-day study with a 28-day recovery period, Crj: CD (SD) IGS rats were exposed to gaseous CPME up to 4 mg/L (6 h/day, 5 days/week) by whole-body inhalation exposure (2). Toxic effects occurred at 4 mg/L and included clinical findings of salivation and nasal discharge, decreased body weights, increased levels of alanine aminotransferase and potassium (in males), increased absolute and body weight-relative kidney weight (in males), hyaline droplets in the proximal tubular epithelium of the kidney, and simple hyperplasia of the mucosal epithelium of the urinary bladder. All adverse effects were reversible following the recovery period. The NOEL of this study was determined to be 0.84 mg/L.
在一起含
28
天恢复期的
90
天研究中,将
Crj: CD (SD) IGS
大鼠全身吸入暴露于
4 mg/L
(
6
小时
/
天,
5
天
/
周)气相
CPME
中。
4 mg/L
浓度时观察到毒性影响,还有临床现象包括流涎和流鼻涕、体重降低、丙氨酸转氨酶与钾(雄鼠)水平增加、绝对和体重相对肾重(雄鼠)增加、肾近端小管上皮玻璃滴状性变,以及膀胱粘膜上皮简单增生。所有不良影响在恢复期后均可逆。该研究中
NOEL
值为
0.84mg/L
。
The most appropriate and well-documented study for CPME toxicity was the 28-day oral rat study.The PDE was calculated based on the identified NOEL of 150 mg/kg/day from this study.
有完整文件记录的最恰当
CPME
毒性研究为
28
天口服大鼠研究。现根据该研究所识别的
NOEL
值
150mg/kg/
天计算
PDE
值。

Conclusion
结论
The calculated PDE for CPME is 15 mg/day based upon the NOEL from the 28-day oral toxicity study. Therefore, it is recommended that CPME be placed into Class 2 “Solvents to Be Limited” in Table 2 in the ICH Impurities: Residual Solvents Guideline.
根据
28
天口服毒性研究所得
NOEL
值,
CPME
计算得
PDE
值为
15mg/
天。因此,建议将
CPME
归为
ICH
杂质
“
残留溶剂指南
”
中表
2
所列
2
类溶剂
“
需限制的溶剂
”
。
References
参考文献
Antonucci V, Coleman J, Ferry JB, Johnson N, Mathe M, Scott JP et al. Toxicological assessment of 2-methyltetrahydrofuran and cyclopentyl methyl ether in support of their use in pharmaceutical chemical process development. Org Process Res Dev 2011;15: 939–41.
支持
2-MTHF
和
CPME
在药物化学工艺开发中的毒性评估
Watanabe K. The toxicological assessment of cyclopentyl methyl ether (CPME) as a green solvent. Molecules. 2013;18:3183-94.
CPME
作为绿色溶剂的毒性评估
CPME Material Safety Data Sheet: URL: https://www.cdhfinechemical.com/images/product/msds/37_916070364_CyclopentylM ethylEther-CASNO-5614-37-9-MSDS.pdf (last accessed on 19 November 2019).
CPME MSDS
Watanabe K, Yamagiwa N, Torisawa Y. Cyclopentyl methyl ether as a new and alternative process solvent. Org. Process Res. Dev. 2007;11:251-58.
CPME
作为新替代工艺溶剂
European Chemicals Agency (ECHA), 2019. Methoxycyclopentane. CASRN 5614-37-9. (last accessed on 19 November 2019). URL: https://echa.europa.eu/registration- dossier/-/registered-dossier/26626/7/9/2
欧洲化学品管理局(
ECHA
),
2019
,环戊基甲醚
Inoue K, Suzuki H, Yamada T. Comprehensive toxicity evaluation of cyclopentyl methyl ether (CPME) for establishing a permitted daily exposure level. Fundam. Toxicol. Sci. 2019;6:145-65.
CPME
建立
PDE
水平的综合毒性评估
TERTIARY-BUTYL ALCOHOL
叔丁醇
Introduction
概述
Tertiary-butyl alcohol (t-Butyl alcohol, tert-butanol; TBA: CAS Number 75-65-0) is a tertiary aliphatic alcohol and used for a variety of purposes including as an alcohol denaturant, a dehydration agent, and a solvent (1). TBA is soluble in water and has a vapour pressure of 31 mm Hg (20°C). TBA is rapidly absorbed following inhalation or ingestion but poorly absorbed through skin (2).
叔丁醇(
2-
甲基
-2-
丙醇、三甲基甲醇、
TBA
,
CAS
号
75-65-0
)为脂肪叔醇,有着广泛用途,包括作为乙醇变性剂、脱水剂和溶剂。
TBA
可溶于水,蒸汽压为
31mmHg
(
20°C
)。
TBA
在吸入或口服后会快速吸收,但皮肤吸收不良。
The rat oral LD50 (lethal dose for 50% of animals, combined values for males and females) has been reported to be between 2733 and 3500 mg/kg body weight. The primary acute effects observed in animals are signs of alcoholic intoxication. Human clinical test data indicate that TBA is neither an irritant nor a sensitizer (3). Its potency for intoxication is approximately 1.5 times that of ethanol(4). Given its wide diversity of use, the potential for human exposure to TBA is high (5). The National Institute for Occupational Safety and Health (NIOSH) indicates its use is widespread in the workplace (1). A Cosmetic Ingredient Review Expert Panel also concluded that TBA is safe as used in cosmetic products (3).
大鼠口服摄入时
LD50
(半致死量,雄性和雌性合并值)有报道为
2733-3500mg/kg
体重。动物中观察到的基本急性影响为酒精中毒的表现。人体临床试验数据显示
BTA
无刺激性,亦不引导起过敏。中毒量约为乙醇的
1.5
倍。考虑到使用范围较广,人体暴露于
TBA
的可能性较高。国家职业安全与健康研究所(
NIOSH
)指出其在工作场所被广泛使用。化妆品成分审评专家组亦得出结论认为
TBA
可安全用于化妆产品。
Genotoxicity
基因毒性
TBA was not mutagenic in the AMES bacterial reverse mutation assay (6). The US National Toxicology Program (NTP) studies also showed TBA was not genotoxic in vitro with and without metabolic activation (S9) (mouse lymphoma cell mutation assay, chromosome aberrations, sister chromatid exchanges). In vivo, no increases in micronucleated erythrocytes were observed in peripheral blood samples from mice administered up to 40000 ppm TBA in drinking water for 13 weeks or up to 625 mg/kg administered by i.p. injection three times at 24- hour intervals (6). In conclusion, there is no evidence that TBA is genotoxic (2).
TBA
在
AMES
细菌反向诱变试验中未显示出诱变性。美国国家毒理部(
NTP
)研究亦显示
TBA
在有代谢活化和无代谢活化(
S9
)(小鼠淋巴瘤细胞突变试验、染色体畸变、姐妹染色单体交换)的体外试验中无基因毒性。小鼠
13
周饮水摄入
40000ppm
,或
24
小时腹腔注射
3
次
625mg/kg
摄入,体内试验中外周血样未观察到微核红细胞增加。总之,无证据证明
TBA
具有基因毒性。
Carcinogenicity
致癌性
TBA was investigated by the US National Toxicology Program (NTP) in two drinking water studies, one in F344/N rats and one in B6C3F1 mice (1,6). Both studies included three treatment groups (60 animals/sex/group; 50 animals/sex/group completed the study): in rats, doses of 85, 195, and 420 mg/kg/day in males and 175, 330, and 650 mg/kg/day in females; in mice, doses of 535, 1035, and 2065 mg/kg/day in males and 510, 1015, and 2105 mg/kg/day in females) (1). Survival was decreased in high dose rats and high dose male mice. Final mean body weights were decreased in exposed male and high dose female rats and high dose female mice. The primary targets of TBA were the kidney (mineralization, hyperplasia, tumours) in male rats and the thyroid gland (follicular cell hyperplasia, tumours) and urinary bladder (inflammation and epithelial hyperplasia) in mice. The NTP Technical Report concluded that there was some evidence of carcinogenic activity in male rats based on increased incidences of renal tubule adenoma or carcinoma (combined) and in female mice based on increased incidences of follicular cell adenoma of the thyroid gland (6).
There was no evidence of carcinogenicity in female rats and equivocal evidence in male mice.
美国国家毒理部(
NTP
)在
TBA
的
2
起饮水给药研究中,一组采用
F344/N
大鼠,另一组采用
B6C3F1
小鼠。两起研究包括
3
个治疗组(
60
只动物
/
性别
/
组,
50
只动物
/
性别
/
组完成了研究):大鼠中,给药量为雄鼠
85
、
195
和
420mg/kg/
天,雌鼠为
175
、
330
和
650 mg/kg/
天;小鼠中,给药量为雄鼠
535
、
1035
和
2065mg/kg/
天,雌鼠为
510
、
1015
和
2105mg/kg/
天。高剂量大鼠和高剂量小雄鼠中存活数均降低。暴露的雄大鼠和高剂量雌性大鼠和高剂量雌性小鼠最终平均体重降低。
TBA
的靶器官对雄大鼠为肾(钙化、细胞增生、肿瘤),对小鼠为甲状腺(滤泡细胞增生、肿瘤)和膀胱(炎症、上皮细胞增生)。
NTP
技术报告结论称有证据证明雄性大鼠和雌性小鼠中有致癌活性,因为在雄性大鼠中观察到肾小管腺瘤或癌变(合并)事件增加,在雌性小鼠中观察到甲状腺滤泡细胞腺瘤事件增加。雌性大鼠中未发现致癌证据,雄性大鼠中证据难解释。
In mice, the incidence of thyroid follicular cell adenoma was significantly increased in high dose females. These tumorigenic effects were associated with an increased incidence and severity of focal follicular cell hyperplasia of the thyroid gland in all TBA-treated groups of males and females (1,6). In contrast, no thyroid tumours were observed in an 18-month carcinogenicity study of methyl tert-butyl ether (MTBE) by the inhalation route in CD-1 mice (7). The systemic TBA exposure (as a metabolite of MTBE) likely exceeded the exposure in the NTP study (2). However, differences in strain of mice (CD-1 versus B6C3F1) or route of administration may be responsible for the differences in response. In the absence of evidence suggesting direct thyroid toxicity, it was hypothesized that TBA induced thyroid tumours in the drinking water study through increased liver metabolism of thyroid hormones, triggering a compensatory increase in thyroid stimulating hormone (TSH) production and, thus, thyroid follicular cell proliferation and hyperplasia (2). Rodents are substantially more sensitive than humans to the development of thyroid follicular cell tumours in response to thyroid hormone imbalance. Thus, the dose response is non-linear and tumours are not expected to occur in humans in the absence of altered thyroid hormone homeostasis (8,9). In partial agreement with the above hypothesis, TBA is an inducer of Phase I and
Ⅱ
liver enzymes following 14 days of oral exposure at doses less than or equal to those used in chronic studies and TBA administration resulted in a small decrease in circulating thyroid hormones in B6C3F1 mice (10). However, no meaningful changes in TSH levels were observed in this study. A comprehensive review of the mouse carcinogenicity data concluded that, in the absence of meaningful effect on TSH and toxicity to the thyroid, the cause of the increase in either hyperplasia or adenoma incidence remains unclear (2). TBA administration also resulted in an increased incidence of chronic inflammation and hyperplasia of the transitional epithelium of the urinary bladder in high-dose males and females.
小鼠中,高剂量雌性甲状腺滤泡细胞腺瘤事件显著增加。这些致瘤影响伴随着所有
BTA
治疗组雄性和雌性甲状腺滤泡细胞腺瘤事件和严重性增加。相反,在
18
个月
CD-1
小鼠吸入
MTBE
致癌性研究中,未发现甲状腺肿瘤。系统性
BTA
暴露(作为
MTBE
的代谢物)可能超出了
NTP
研究中的暴露量。当然,小鼠(
CD-1 VS B6C3F1
)压力差异或摄入方式差异可能会导致响应差异。因为无证据显示直接甲状腺毒性,因此假设饮水研究里的
TBA
通过增加甲状腺激素的肝代谢物,诱发了甲状腺肿瘤,激发促甲状腺激素(
TSH
)分泌的补偿性增加,从而产生甲状腺滤泡细胞增殖和细胞增生。啮齿动物在甲状腺激素不平衡时产生甲状腺滤泡细胞增殖方面比人类敏感许多。因此,对剂量的响应并不是线性的,在人类中没有改变甲状腺激素体内稳定状态时不会引发肿瘤。部分同意上述假设情况下,在低于或等于长期研究中所用剂量下口服摄入
14
天后,
BTA
会诱导生成一期和二期肝酶,
TBA
摄入导致
B6C3F1
小鼠中循环甲状腺激素小量降低。但是在该研究中未发现有实质意义的
TSH
水平改变。对小鼠致癌性数据审核结论为,由于
TSH
和甲状腺毒性无实质意义的影响,因此细胞增生或腺瘤事件增加的原因不明。
TBA
摄入亦导致高剂量雄性和雌性鼠膀胱上皮长期炎症和细胞增生事件增加。
In rats, an increased incidence of renal tubule adenomas and carcinomas was observed in males exposed to TBA, but the increase was not dose-dependent. The evidence suggests that these tumours are due to a α2µ-globulin nephropathy-mediated mode of action. α2µ-Globulin nephropathy is a well-recognized sex- and species-specific mechanism of toxicity without relevance to humans (11,12). Foci of linear mineralization in the renal medulla, a lesion consistently reported as a long-term consequence of α2µ-globulin nephropathy, were observed in the high dose male rats (1,6). Further, TBA was shown to interact with α2µ, which explains the accumulation of α2µ in the male rat kidney (5). Although no significant neoplastic findings were observed in female rats, a dose-dependent increase in severity of nephropathy was observed at all TBA doses compared to control animals (average severity of 1.6, 1.9, 2.3, and 2.9; scale of 0–4); incidence ranged from 47–48 out of 50 animals in all groups. An increased incidence of transitional epithelial hyperplasia and suppurative inflammation at the two highest doses and renal tubule hyperplasia in a single high dose animal were also observed. The human relevance of the renal findings in female rats is currently unclear.
大鼠试验中,暴露于
TBA
雄性大鼠观察到肾小管腺瘤和癌发生事件增加,但增加程度与剂量无关。证据显示这些肿瘤是由于
α2µ-
球蛋白肾病中介作用方式。
α2µ-
球蛋白肾病是一种已有深入认识的有性别和物种选择性的毒性机制,与人类无关。高剂量雄性大鼠中观察到长期
α2µ-
球蛋白肾病引发肾髓质线性钙化中心的损伤的报道比较一致。另外,
TBA
显示出与
α2µ
有相互作用,这解释了
α2µ
在雄性大鼠肾中的累积情况。虽然在雌性小鼠中未观察到显著肿瘤情况,但在所有
TBA
剂量组均观察到相对于对照组的肾病严重程度增加(平均严重程度分为
1.6
、
1.9
、
2.3
和
2.9
,分级为
0-4
),与剂量无关;所有组中
50
只动物有
47-48
只发生。
2
组最高剂量组中观察到移行上皮增生和化脓性炎症,在单个高剂量动物中观察到肾小管增生。雌性大鼠中发现的肾脏问题与人类相关性尚不明确。
The 2-year carcinogenicity studies were considered the most relevant for calculation of the PDE for TBA. From the results of the rat and mouse carcinogenicity studies, PDEs were calculated based on two different scenarios:
2
年致癌研究被认为是与
TBA
的
PDE
计算相关性最高。从大鼠与小鼠的致癌研究结果,可基于以下
2
种情形计算
PDE
值:
renal lesions and tumour findings in male rats are not relevant to humans and, therefore, the increased severity in nephropathy observed in female rats at the lowest dose (LOEL = 175 mg/kg/day) is used for the PDE calculation.
雄性大鼠中观察到的肾脏损伤和肿瘤与人类无关,因此在最低剂量的雌性大鼠中观察到的肾病严重程度的增加(
LOEL=175mg/kg/
天)被用于
PDE
计。
Or
或
increased incidence of follicular cell hyperplasia in the thyroid of female mice at the lowest TBA dose (LOEL = 510 mg/kg/day) is used for the PDE calculation.
最低
TBA
剂量(
LOEL=510mg/kg/
天)雌性小鼠甲状腺滤泡细胞增生事件增加被用于
PDE
计算。
Scenario 1 (rat): LOEL(nephropathy) 175 mg/kg/day
1
种情形(大鼠):
LOEL
(肾病)
175 mg/kg/
天
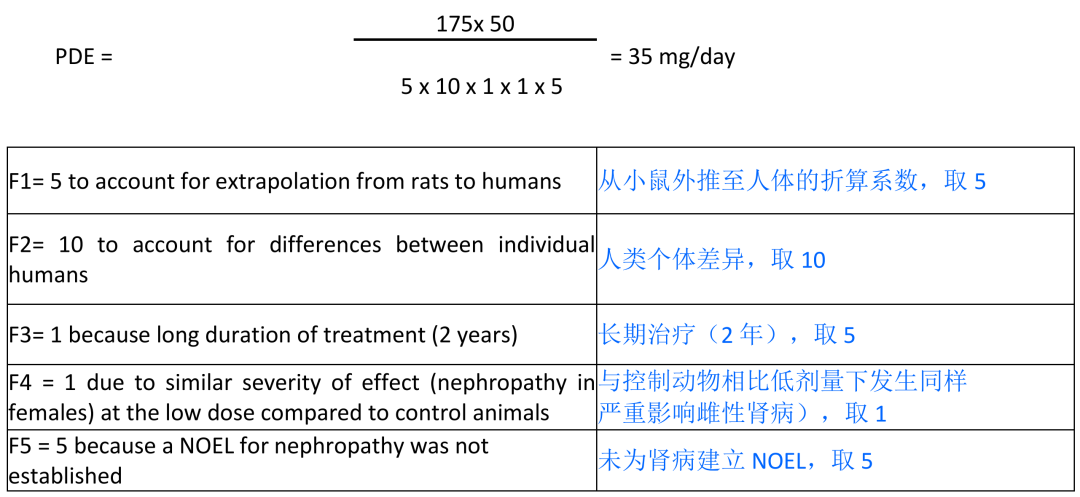
Limit
限度
= (35 1000)/10 = 3500 ppm
Scenario 2 (mouse): LOEL(follicular cell hyperplasia) 510 mg/kg/day
2
种情形(小鼠):
LOEL
(滤泡细胞增生)
510 mg/kg/day
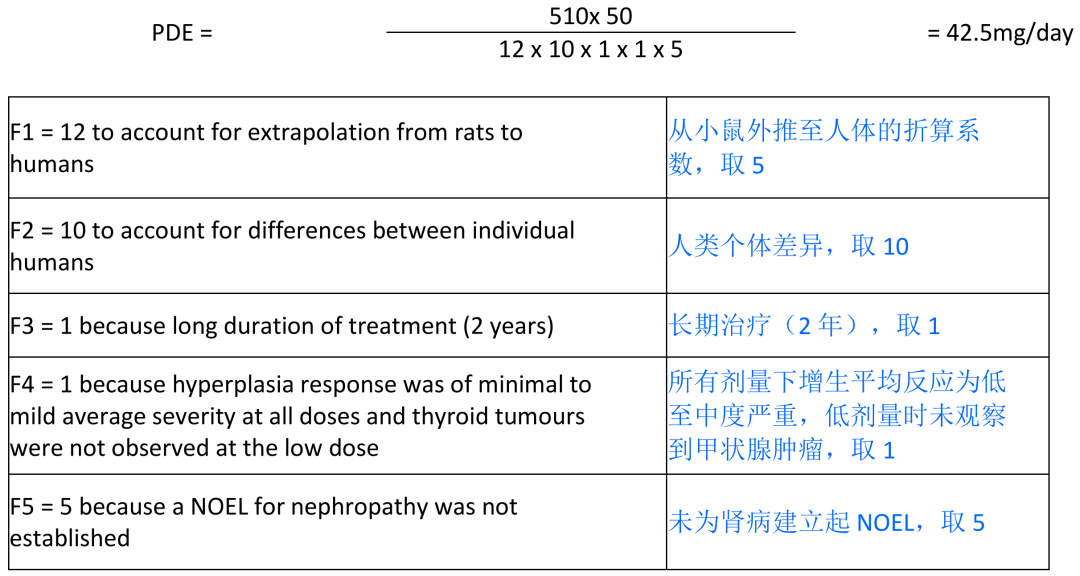
Limit
限度
= (42.5 1000)/10 = 4250 ppm
The ultimate PDE for TBA, calculated based on the identified LOEL of 175 mg/kg/day from 2- year rat study, is 35 mg/day.
根据
2
年大鼠研究所确定的
LOEL
(
175 mg/kg/day
)计算,
TBA
最终
PDE
值定为
35mg/
天。
Reproductive toxicity
生殖毒性
TBA has not been associated with induction of skeletal or visceral malformations in rats or mice but did induce developmental delays and intrauterine or prenatal mortality at doses of 1000 mg/kg/day or greater (2).
TBA
与小鼠和大鼠骨骼或内脏畸形诱发未建立关联性,但确实在
1000mg/kg/
天或更高剂量时诱发发育推迟和子宫内或产前死亡。
In a reproduction/developmental toxicity screening study, TBA was administered to Sprague-Dawley rats (12/sex/group) by oral gavage at dose levels of 0, 64, 160, 400, and 1000 mg/kg/day for up to 63 days in males and from 4 weeks prior to mating until postnatal day (PND) 20 in females (13). There were no adverse effects on any reproductive parameters including mating index, fertility index, pregnancy index, or gestation index. For dams receiving 1000 mg/kg/day TBA through gestation and lactation, there was a significant reduction in mean litter size, a decrease in the number of live born per pregnancy, an increase in the number of stillborn pups, increased pup mortality up to PND 4, and a decrease in mean pup body weight at birth, which continued to weaning. Parental toxicity (transient CNS effects, reduced body weight and food consumption) was observed at doses of 400 mg/kg or greater. The NOAEL for developmental/reproductive effects was identified as 400 mg/kg/day.
在一起生殖
/
发育毒性筛选研究中,斯泼累格
·
多雷雄性大鼠(
12/
性别
/
组)口服
TBA0
、
64
、
160
、
400
和
1000 mg/kg/
天饲喂















